StemSpan™ NK Cell Generation Kit
StemSpan™ Lymphoid Progenitor Expansion Supplement (10X) contains a combination of recombinant human cytokines and other additives formulated to selectively promote the expansion and differentiation of CD34+ cells isolated from human CB and BM samples to lymphoid progenitor cells when used in combination with StemSpan™ SFEM II medium, and on plates coated with StemSpan™ Lymphoid Differentiation Coating Material (100X). Subsequently, StemSpan™ NK Cell Differentiation Supplement (100X) enables differentiation of lymphoid progenitor cells to NK cells.
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Product Information Sheet | StemSpan™ SFEM II | 09605 | All | English |
| Product Information Sheet | StemSpan™ Lymphoid Progenitor Expansion Supplement (10X) | 09915 | All | English |
| Product Information Sheet | StemSpan™ Lymphoid Differentiation Coating Material (100X) | 09925 | All | English |
| Product Information Sheet | StemSpan™ NK Cell Differentiation Supplement (100X) | 09950 | All | English |
| Manual | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Safety Data Sheet 1 | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Safety Data Sheet 2 | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Safety Data Sheet 3 | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Safety Data Sheet 4 | StemSpan™ NK Cell Generation Kit | 09960 | All | English |
| Safety Data Sheet | StemSpan™ SFEM II | 09605 | All | English |
Data
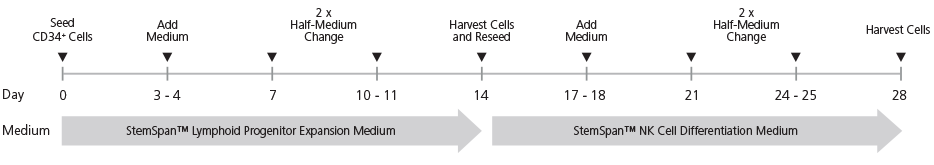
Figure 1. StemSpan™ NK Cell Generation Protocol
CB-derived CD34+ cells are seeded on day 0. Medium should be topped up after 3 - 4 days of culture followed by two half-medium changes every 3 - 4 days. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded for further differentiation into NK cells. Top-up and half-medium changes should be performed every 3 - 4 days after harvest and reseed, as indicated in the figure. Note: UM729 should only be added to the NK Cell Differentiation Medium, but not the Lymphoid Progenitor Expansion Medium.
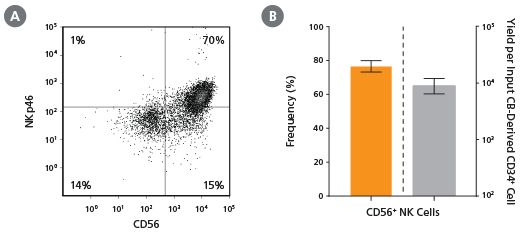
Figure 2. Frequency and Yield of CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells (freshly isolated or frozen) were cultured with the StemSpan™ NK Cell Generation Kit for 28 days as described. Cells were harvested and analyzed for (A,B) CD56 and (A) NKp46 expression by flow cytometry. Dead cells were excluded by light scatter profile and viability staining. (B) The average frequency of viable CD56+ NK cells on day 28 was 77%, with ~9,000 CD56+ cells produced per input CB-derived CD34+ cell. Shown are means with 95% confidence intervals (n = 45: 23 freshly isolated and 22 frozen CD34+ cell samples). BM-derived CD34+ cells were also differentiated into NK cells using the StemSpan™ NK Cell Generation Kit. The yield of NK cells from BM HSPCs is typically lower than with CB, averaging ~75 per input CD34+ cell (n = 3, data not shown).
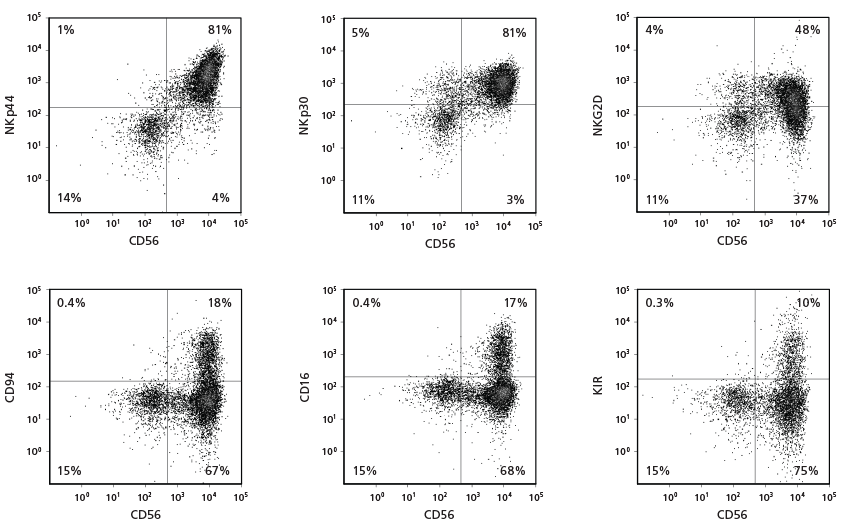
Figure 3. Cell Surface Marker Expression on CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells were cultured with the StemSpan™ NK Cell Generation Kit for 28 days. The differentiated cells were harvested and analysed by flow cytometry for the expression of CD56, NKp44, NKp30, NKG2D, CD94, CD16, and KIR. Staining for KIR molecules was performed using a combination of two clones for the antibody, 180704 and HP-MA4, as each recognizes a distinct subset of KIR molecules.
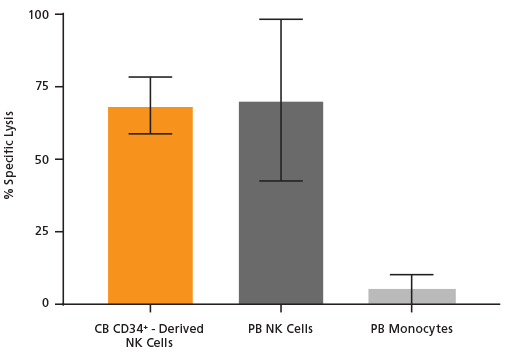
Figure 4. Cultured NK Cells Exhibit Cytotoxicity Toward K562 Cell Line
NK cells were generated from CB-derived CD34+ cells over 28 days using the protocol in Figure 1. On day 28, cells were harvested, stained for CD56, and viable CD56+ cells were counted. K562 cells were incubated with 8 μM calcein AM at 37°C for 1 hour and then washed twice. CD56+ NK cells were then combined with 10,000 of these calcein AM-labeled K562 target cells at an Effector:Target ratio of 5:1 in U-bottom 96-well plates and co-cultured at 37°C for 4 hours. Adult peripheral blood (PB) NK cells and monocytes isolated using EasySep™ were used as positive and negative controls, respectively. PB NK cells were cultured overnight with the NK Cell Differentiation Supplement and SFEM II, while PB monocytes were cultured overnight in SFEM II only. To detect spontaneous release, control wells containing only calcein AM-labeled K562 target cells were set up. The labeled K562 cells were treated with 1% Triton™ X-100 to measure maximum release. After incubation, plates were centrifuged at 500 x g for 5 minutes and 100 μL of supernatant was transferred to black plates and analyzed using a SpectraMax® microplate reader (excitation 485 nm/emission 530 nm). Results are expressed as % specific lysis: [(test release - spontaneous release) x 100] / (maximum release - spontaneous release). CB CD34+-derived NK cells show similar killing activity toward K562 target cells compared to PB NK cells. Shown are means ± SD (CB CD34+-derived NK cells: n = 18, PB NK cells and monocytes: n = 7).