StemSpan™ Megakaryocyte Expansion Supplement (100X)
Serum-free culture supplement for expansion of human megakaryocytes
概要
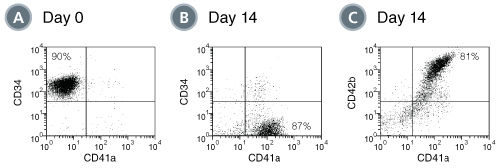
StemSpan™ Megakaryocyte Expansion Supplement (100X) contains a combination of recombinant human cytokines formulated to selectively promote the expansion and differentiation of human megakaryocyte progenitor cells from CD34+ cells isolated from human cord blood (CB) or bone marrow (BM) samples.
StemSpan™ Megakaryocyte Expansion Supplement (100X) is intended for use in combination with any of the following StemSpan™ media:
• StemSpan™ SFEM (Catalog #09600)
• StemSpan™ SFEM II (Catalog #09605)
• StemSpan™-XF (Catalog #100-0073)
• StemSpan™-AOF (Catalog #100-0130)
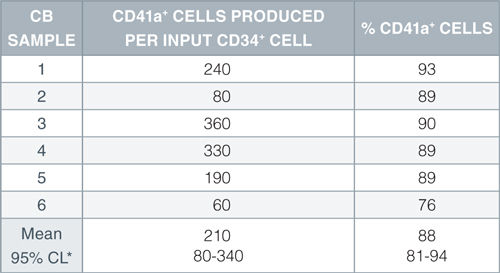
When added to serum-free medium, StemSpan™ Megakaryocyte Expansion Supplement typically promotes the production of hundreds of megakaryocytes per input CD34+ cell in 14-day liquid cultures initiated with CD34+ human CB cells. See data tab for more details.
• Formulated to produce large numbers of human megakaryocytes in liquid cultures initiated with CD34+ CB or BM cells.
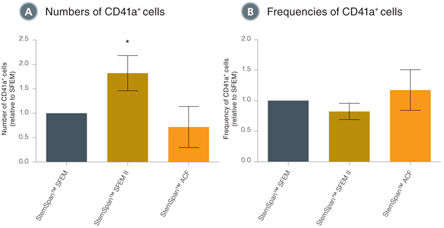
• Optimized for use with StemSpan™ media. When combined with StemSpan™ SFEM II in particular, supports up to 2-fold higher expansion of megakaryocytes from human CD34+ CB cells than other serum-free media on the market.
• Supplied as a 100X concentrate. After thawing and mixing, the tube contents can be added directly to any hematopoietic cell expansion medium of choice.
• Recombinant human stem cell factor (SCF)
• Recombinant human interleukin 6 (IL-6)
• Recombinant human interleukin 9 (IL-9)
• Recombinant human thrombopoietin (TPO)
Hematopoietic Stem and Progenitor Cells, Megakaryocytes
Cell Culture, Differentiation, Expansion
Stem Cell Biology, Transplantation Research
数据及文献
Publications (12)
Blood 2017 MAR
Identification of unipotent megakaryocyte progenitors in human hematopoiesis.
Miyawaki K et al.
Abstract
The developmental pathway for human megakaryocytes remains unclear and the definition of pure unipotent megakaryocyte progenitor is still controversial. Using single-cell transcriptome analysis, we have identified a cluster of cells within immature hematopoietic stem and progenitor cell populations that specifically express genes related to the megakaryocyte lineage. We used CD41 as a positive marker to identify these cells within the CD34(+)CD38(+)IL-3Rα(dim)CD45RA(-) common myeloid progenitor (CMP) population. These cells lacked erythroid and granulocyte/macrophage potential, but exhibited robust differentiation into the megakaryocyte lineage at a high frequency, both in vivo and in vitro The efficiency and expansion potential of these cells exceeded those of conventional bipotent megakaryocyte/erythrocyte progenitors. Accordingly, the CD41(+) CMP was defined as a unipotent megakaryocyte progenitor (MegP) that is likely to represent the major pathway for human megakaryopoiesis, independent of canonical megakaryocyte-erythroid lineage bifurcation. In the bone marrow of patients with essential thrombocythemia, the MegP population was significantly expanded in the context of a high burden of Janus kinase 2 mutations. Thus, the prospectively isolatable and functionally homogeneous human MegP will be useful for the elucidation of the mechanisms underlying normal and malignant human hematopoiesis.
Stem Cell Reports 2014 NOV
Scalable generation of universal platelets from human induced pluripotent stem cells
Feng Q et al.
Abstract
Human induced pluripotent stem cells (iPSCs) provide a potentially replenishable source for the production of transfusable platelets. Here, we describe a method to generate megakaryocytes (MKs) and functional platelets from iPSCs in a scalable manner under serum/feeder-free conditions. The method also permits the cryopreservation of MK progenitors, enabling a rapid surge" capacity when large numbers of platelets are needed. Ultrastructural/morphological analyses show no major differences between iPSC platelets and human blood platelets. iPSC platelets form aggregates�
Blood 2009 SEP
miR-34a contributes to megakaryocytic differentiation of K562 cells independently of p53.
Navarro F et al.
Abstract
The role of miRNAs in regulating megakaryocyte differentiation was examined using bipotent K562 human leukemia cells. miR-34a is strongly up-regulated during phorbol ester-induced megakaryocyte differentiation, but not during hemin-induced erythrocyte differentiation. Enforced expression of miR-34a in K562 cells inhibits cell proliferation, induces cell-cycle arrest in G(1) phase, and promotes megakaryocyte differentiation as measured by CD41 induction. miR-34a expression is also up-regulated during thrombopoietin-induced differentiation of CD34(+) hematopoietic precursors, and its enforced expression in these cells significantly increases the number of megakaryocyte colonies. miR-34a directly regulates expression of MYB, facilitating megakaryocyte differentiation, and of CDK4 and CDK6, to inhibit the G(1)/S transition. However, these miR-34a target genes are down-regulated rapidly after inducing megakaryocyte differentiation before miR-34a is induced. This suggests that miR-34a is not responsible for the initial down-regulation but may contribute to maintaining their suppression later on. Previous studies have implicated miR-34a as a tumor suppressor gene whose transcription is activated by p53. However, in p53-null K562 cells, phorbol esters induce miR-34a expression independently of p53 by activating an alternative phorbol ester-responsive promoter to produce a longer pri-miR-34a transcript.
Stem cells (Dayton, Ohio) 2007 JAN
In vitro expanded cells contributing to rapid severe combined immunodeficient repopulation activity are CD34+38-33+90+45RA-.
Vanheusden K et al.
Abstract
Expansion of hematopoietic stem cells could be used clinically to shorten the prolonged aplastic phase after umbilical cord blood (UCB) transplantation. In this report, we investigated rapid severe combined immunodeficient (SCID) repopulating activity (rSRA) 2 weeks after transplantation of CD34(+) UCB cells cultured with serum on MS5 stromal cells and in serum- and stroma-free cultures. Various subpopulations obtained after culture were studied for rSRA. CD34(+) expansion cultures resulted in vast expansion of CD45(+) and CD34(+) cells. Independent of the culture method, only the CD34(+)33(+)38(-) fraction of the cultured cells contained rSRA. Subsequently, we subfractionated the CD34(+)38(-) fraction using stem cell markers CD45RA and CD90. In vitro differentiation cultures showed CD34(+) expansion in both CD45RA(-) and CD90(+) cultures, whereas little increase in CD34(+) cells was observed in both CD45RA(+) and CD90(-) cultures. By four-color flow cytometry, we could demonstrate that CD34(+)38(-)45RA(-) and CD34(+)38(-)90(+) cell populations were largely overlapping. Both populations were able to reconstitute SCID/nonobese diabetic mice at 2 weeks, indicating that these cells contained rSRA activity. In contrast, CD34(+)38(-)45RA(+) or CD34(+)38(-)90(-) cells contributed only marginally to rSRA. Similar results were obtained when cells were injected intrafemorally, suggesting that the lack of reconstitution was not due to homing defects. In conclusion, we show that after in vitro expansion, rSRA is mediated by CD34(+)38(-)90(+)45RA(-) cells. All other cell fractions have limited reconstitutive potential, mainly because the cells have lost stem cell activity rather than because of homing defects. These findings can be used clinically to assess the rSRA of cultured stem cells.
Stem cells (Dayton, Ohio) 2006 OCT
Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution.
Goussetis E et al.
Abstract
Central issues in intracoronary infusion (ICI) of bone marrow (BM)-cells to damaged myocardium for improving cardiac function are the cell number that is feasible and safe to be administrated as well as the retention of cells in the target area. Our study addressed these issues in eight patients with chronic ischemic cardiomyopathy undergoing ICI of selected BM-progenitors. We could immunomagnetically isolate 0.8 +/- 0.32 x 10(7) CD133(+) cells and 0.75 +/- 0.24 x 10(7) CD133(-)CD34(+) cells from 310 +/- 40 ml BM. After labeling these cells with (99m)Tc-hexamethylpropylenamineoxime, they were infused into the infarct-related artery without any complication. Scintigraphic images 1 (eight patients) and 24 hours (four patients) after ICI revealed an uptake of 9.2% +/- 3.6 and 6.8% +/- 2.4 of the total infused radioactivity in the infarcted area of the heart, respectively; the remaining activity was distributed mainly to liver and spleen. We conclude that through ICI of CD133(+) and CD133(-)CD34(+) BM-progenitors a significant number of them are preferentially attracted to and retained in the chronic ischemic myocardium.
Blood 1998 MAY
Efficient retroviral-mediated gene transfer to human cord blood stem cells with in vivo repopulating potential.
Conneally E et al.
Abstract
Recent studies have shown efficient gene transfer to primitive progenitors in human cord blood (CB) when the cells are incubated in retrovirus-containing supernatants on fibronectin-coated dishes. We have now used this approach to achieve efficient gene transfer to human CB cells with the capacity to regenerate lymphoid and myeloid progeny in nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice. CD34(+) cell-enriched populations were first cultured for 3 days in serum-free medium containing interleukin-3 (IL-3), IL-6, granulocyte colony-stimulating factor, Flt3-ligand, and Steel factor followed by two 24-hour incubations with a MSCV-NEO virus-containing medium obtained under either serum-free or serum-replete conditions. The presence of serum during the latter 2 days made no consistent difference to the total number of cells, colony-forming cells (CFC), or long-term culture-initiating cells (LTC-IC) recovered at the end of the 5-day culture period, and the cells infected under either condition regenerated similar numbers of human CD34(+) (myeloid) CFC and human CD19(+) (B lymphoid) cells for up to 20 weeks in NOD/SCID recipients. However, the presence of serum increased the viral titer in the producer cell-conditioned medium and this was correlated with a twofold to threefold higher efficiency of gene transfer to all progenitor types. With the higher titer viral supernatant, 17% +/- 3% and 17% +/- 8%, G418-resistant in vivo repopulating cells and LTC-IC were obtained. As expected, the proportion of NEO + repopulating cells determined by polymerase chain reaction analysis of in vivo generated CFC was even higher (32% +/- 10%). There was no correlation between the frequency of gene transfer to LTC-IC and colony-forming unit-granulocyte-macrophage (CFU-GM), or to NOD/SCID repopulating cells and CFU-GM (r2 = 0.16 and 0.17, respectively), whereas values for LTC-IC and NOD/SCID repopulating cells were highly and significantly correlated (r2 = 0.85). These findings provide further evidence of a close relationship between human LTC-IC and NOD/SCID repopulating cells (assessed using a textgreater/= 6-week CFC output endpoint) and indicate the predictive value of gene transfer measurements to such LTC-IC for the design of clinical gene therapy protocols.
View All Publications