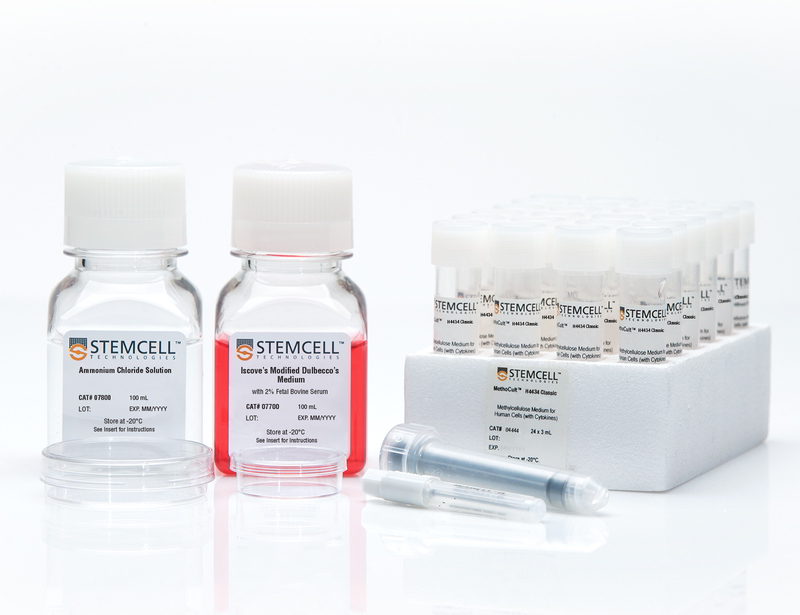
Starter Kit for MethoCult™ H4434 Classic
Complete kit for hematopoietic CFU assays
概要
The Starter Kit for MethoCult™ H4434 Classic (MethoCult™ GF H4434) is recommended for laboratories in the initial stages of establishing procedures for assessing human hematopoietic progenitor cells using colony-forming unit (CFU) assays. These products support the growth of clonogenic hematopoietic progenitor cells from human bone marrow, peripheral blood, cord blood, leukapheresis products, and purified progenitor-enriched cells. H4434 is a complete methylcellulose-based medium that supports the growth of erythroid progenitors (BFU-E and CFU-E), granulocyte-macrophage progenitors (CFU-GM, CFU-G and CFU-M) and multi-potential granulocyte, erythroid, macrophage, megakaryocyte progenitors (CFU-GEMM). Each kit contains instructional materials in addition to all reagents and materials necessary to perform 24 duplicate assays.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
Components
- Starter Kit for MethoCult™ H4434 Classic (Catalog #04464)
- MethoCult™ H4434 Classic, 24 x 3 mL tubes (Catalog #04444)
- Iscove's Modified Dulbecco's Medium (IMDM) with 2% Fetal Bovine Serum, 100 mL (Catalog #07700)
- Ammonium Chloride Solution, 100 mL (Catalog #07800)
- 35 mm Culture Dishes, 10/pack (Catalog #27100)
- 60 mm Gridded Scoring Dishes, 20/pack (Catalog #100-0085)
- Blunt-End Needles, 30/bag (Catalog #28130)
- 3 cc Syringes, 30/bag (Catalog #28230)
- Colony Atlas (Catalog #28700)
Subtype
Semi-Solid Media, Specialized Media
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Human, Non-Human Primate
Application
Cell Culture, Colony Assay, Functional Assay
Brand
MethoCult
Area of Interest
Stem Cell Biology
技术资料
数据及文献
Data
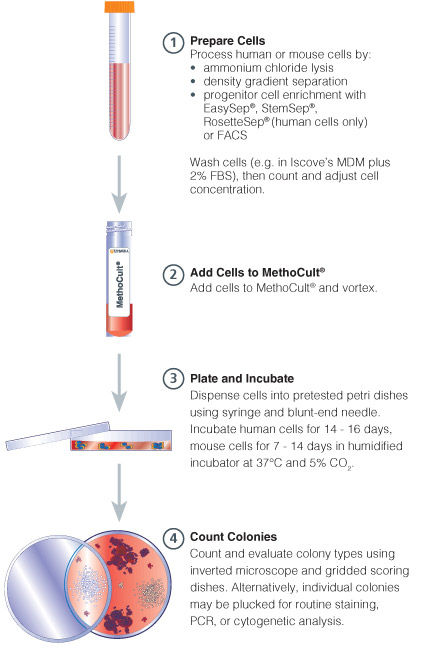
Figure 1. Procedure Summary for Hematopoietic CFU Assays
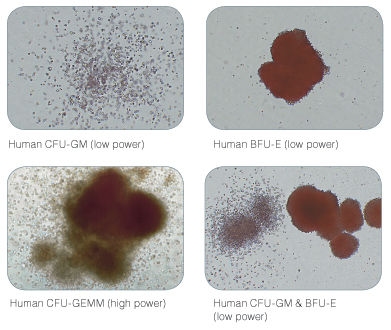
Figure 2. Examples of Colonies Derived from Human Hematopoietic Progenitors
Publications (7)
Nature Communications 2015 JAN
Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6
Abstract
Abstract
Human induced pluripotent stem cells (hiPSCs) have potential for personalized and regenerative medicine. While most of the methods using these cells have focused on deriving homogenous populations of specialized cells, there has been modest success in producing hiPSC-derived organotypic tissues or organoids. Here we present a novel approach for generating and then co-differentiating hiPSC-derived progenitors. With a genetically engineered pulse of GATA-binding protein 6 (GATA6) expression, we initiate rapid emergence of all three germ layers as a complex function of GATA6 expression levels and tissue context. Within 2 weeks we obtain a complex tissue that recapitulates early developmental processes and exhibits a liver bud-like phenotype, including haematopoietic and stromal cells as well as a neuronal niche. Collectively, our approach demonstrates derivation of complex tissues from hiPSCs using a single autologous hiPSCs as source and generates a range of stromal cells that co-develop with parenchymal cells to form tissues.
Blood 2000 JAN
High levels of lymphoid expression of enhanced green fluorescent protein in nonhuman primates transplanted with cytokine-mobilized peripheral blood CD34(+) cells.
Abstract
Abstract
We have used a murine retrovirus vector containing an enhanced green fluorescent protein complimentary DNA (EGFP cDNA) to dynamically follow vector-expressing cells in the peripheral blood (PB) of transplanted rhesus macaques. Cytokine mobilized CD34(+) cells were transduced with an amphotropic vector that expressed EGFP and a dihydrofolate reductase cDNA under control of the murine stem cell virus promoter. The transduction protocol used the CH-296 recombinant human fibronectin fragment and relatively high concentrations of the flt-3 ligand and stem cell factor. Following transplantation of the transduced cells, up to 55% EGFP-expressing granulocytes were obtained in the peripheral circulation during the early posttransplant period. This level of myeloid marking, however, decreased to 0.1% or lower within 2 weeks. In contrast, EGFP expression in PB lymphocytes rose from 2%-5% shortly following transplantation to 10% or greater by week 5. After 10 weeks, the level of expression in PB lymphocytes continued to remain at 3%-5% as measured by both flow cytometry and Southern blot analysis, and EGFP expression was observed in CD4(+), CD8(+), CD20(+), and CD16/56(+) lymphocyte subsets. EGFP expression was only transiently detected in red blood cells and platelets soon after transplantation. Such sustained levels of lymphocyte marking may be therapeutic in a number of human gene therapy applications that require targeting of the lymphoid compartment. The transient appearance of EGFP(+) myeloid cells suggests that transduction of a lineage-restricted myeloid progenitor capable of short-term engraftment was obtained with this protocol. (Blood. 2000;95:445-452)
Experimental hematology 1999 NOV
Comparison of in vitro drug-sensitivity of human granulocyte-macrophage progenitors from two different origins: umbilical cord blood and bone marrow.
Abstract
Abstract
Predictive in vitro hematotoxicity assays using human cells will provide estimation of tolerable level and aid considerably the development of agents with greater therapeutic activity and less toxicity. Human hematopoietic cells can be derived from three sources: human bone marrow by sternal or femoral aspiration, mobilized peripheral blood, or umbilical cord blood samples collected from placentas after deliveries. Because of the difficulties to have a continuous supply of bone marrow cells from normal human donors and the related ethical problems, we performed a study to compare the sensitivity of human bone marrow cells (h-BMC) and human cord blood cells (h-CBC) to chemicals in order to confirm if h-CBC can readily replace bone marrow cells in checking the sensitivity of GM-CFU progenitors to drugs as preliminarily reported in literature. Our results showed that the prediction of IC50 values in human model is quite similar by using h-BMC or h-CBC. On the contrary, the type of medium influenced in a significant way the ICs determination of some drugs.
Blood 1996 JAN
Acceleration of hematopoietic reconstitution with a synthetic cytokine (SC-55494) after radiation-induced bone marrow aplasia.
Abstract
Abstract
The synthetic cytokine (Synthokine) SC-55494 is a high-affinity interleukin-3 (IL-3) receptor ligand that stimulates greater in vitro multilineage hematopoietic activity than native IL-3, while inducing no significant increase in inflammatory activity relative to native IL-3. The aim of this study was to investigate the in vivo hematopoietic response of rhesus monkeys receiving Synthokine after radiation-induced marrow aplasia. Administration schedule and dose of Synthokine were evaluated. All animals were total-body irradiated (TBI) with 700 cGy 60Co gamma radiation on day 0. Beginning on day 1, cohorts of animals (n = 5) received Synthokine subcutaneously (SC) twice daily with 25 micrograms/kg/d or 100 micrograms/kg/d for 23 days or 100 micrograms/kg/d for 14 days. Control animals (n = 9) received human serum albumin SC once daily at 15 micrograms/kg/d for 23 days. Complete blood counts were monitored for 60 days postirradiation and the durations of neutropenia (NEUT; absolute neutrophil count [ANC] textless 500/microL) and thrombocytopenia (THROM; platelet count textless 20,000/microL) were assessed. Synthokine significantly (P textless .05) reduced the duration of THROM versus the HSA-treated animals regardless of dose or protocol length. The most striking reduction was obtained in the animals receiving 100 micrograms/kg/d for 23 days (THROM = 3.5 v 12.5 days in HSA control animals). Although the duration of NEUT was not significantly altered, the depth of the nadir was significantly lessened in all animal cohorts treated with Synthokine regardless of dose versus schedule length. Bone marrow progenitor cell cultures indicated a beneficial effect of Synthokine on the recovery of granulocyte-macrophage colony-forming units that was significantly higher at day 24 post-TBI in both cohorts treated at 25 and 100 micrograms/kg/d for 23 days relative to the control animals. Plasma pharmacokinetic parameters were evaluated in both normal and irradiated animals. Pharmacokinetic analysis performed in irradiated animals after 1 week of treatment suggests an effect of repetitive Synthokine schedule and/or TBI on distribution and/or elimination of Synthokine. These data show that the Synthokine, SC55 94, administered therapeutically post-TBI, significantly enhanced platelet recovery and modulated neutrophil nadir and may be clinically useful in the treatment of the myeloablated host.
Blood 1996 JAN
Rapid and efficient selection of human hematopoietic cells expressing murine heat-stable antigen as an indicator of retroviral-mediated gene transfer.
Abstract
Abstract
Recombinant retroviruses offer many advantages for the genetic modification of human hematopoietic cells, although their use in clinical protocols has thus far given disappointing results. There is therefore an important need to develop new strategies that will allow effectively transduced primitive hematopoietic target populations to be both rapidly characterized and isolated free of residual nontransduced but biologically equivalent cells. To address this need, we constructed a murine stem cell virus (MSCV)-based retroviral vector containing the 228-bp coding sequence of the murine heat-stable antigen (HSA) and generated helper virus-free amphotropic MSCV-HSA producer cells by transfection of GP-env AM12 packaging cells. Light density and, in some cases, lineage marker-negative (lin-) normal human marrow or mobilized peripheral blood cells preactivated by exposure to interleukin-3 (IL-3), IL-6, and Steel factor in vitro for 48 hours were then infected by cocultivation with these MSCV-HSA producer cells for a further 48 hours in the presence of the same cytokines. Fluorescence-activated cell sorting (FACS) analysis of the cells 24 hours later showed 21% to 41% (mean, 27%) of those that were still CD34+ to have acquired the ability to express HSA. The extent of gene transfer to erythroid and granulopoietic progenitors (burst-forming unit-erythroid and colony-forming unit-granulocyte-macrophage), as assessed by the ability of these cells to form colonies of mature progeny in the presence of normally toxic concentrations of G418, averaged 11% and 12%, respectively, in 6 experiments. These values could be increased to 100% and 77%, respectively, by prior isolation of the CD34+HSA+ cell fraction and were correspondingly decreased to an average of 2% and 5%, respectively, in the CD34+HSA- cells. In addition, the extent of gene transfer to long-term culture-initiating cells (LTC-IC) was assessed by G418 resistance. The average gene transfer to LTC-IC-derived colony-forming cells in the unsorted population was textless or = 7% in 4 experiments. FACS selection of the initially CD34+HSA+ cells increased this value to 86% and decreased it to 3% for the LTC-IC plated from the CD34+HSA- cells. Transfer of HSA gene expression to a phenotypically defined more primitive subpopulation of CD34+ cells, ie, those expressing little or no CD38, could also be shown by FACS analysis of infected populations 24 hours after infection. These findings underscore the potential use of retroviral vectors encoding HSA for the specific identification and non-toxic selection immediately after infection of retrovirally transduced populations of primitive human hematopoietic cells. In addition, such vectors should facilitate the subsequent tracking of their marked progeny using multiparameter flow cytometry.
Proceedings of the National Academy of Sciences of the United States of America 1996 FEB
Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium.
Abstract
Abstract
A major goal of experimental and clinical hematology is the identification of mechanisms and conditions that support the expansion of transplantable hematopoietic stem cells. In normal marrow, such cells appear to be identical to (or represent a subset of) a population referred to as long-term-culture-initiating cells (LTC-ICs) so-named because of their ability to produce colony-forming cell (CFC) progeny for textgreater or = 5 weeks when cocultured with stromal fibroblasts. Some expansion of LTC-ICs in vitro has recently been described, but identification of the factors required and whether LTC-IC self-renewal divisions are involved have remained unresolved issues. To address these issues, we examined the maintenance and/or generation of LTC-ICs from single CD34+ CD38- cells cultured for variable periods under different culture conditions. Analysis of the progeny obtained from cultures containing a feeder layer of murine fibroblasts engineered to produce steel factor, interleukin (IL)-3, and granulocyte colony-stimulating factor showed that approximately 20% of the input LTC-ICs (representing approximately 2% of the original CD34+ CD38- cells) executed self-renewal divisions within a 6-week period. Incubation of the same CD34+ CD38- starting populations as single cells in a defined (serum free) liquid medium supplemented with Flt-3 ligand, steel factor, IL-3, IL-6, granulocyte colony-stimulating factor, and nerve growth factor resulted in the proliferation of initial cells to produce clones of from 4 to 1000 cells within 10 days, approximately 40% of which included textgreater or = 1 LTC-IC. In contrast, in similar cultures containing methylcellulose, input LTC-ICs appeared to persist but not divide. Overall the LTC-IC expansion in the liquid cultures was 30-fold in the first 10 days and 50-fold by the end of another 1-3 weeks. Documentation of human LTC-IC self-renewal in vitro and identification of defined conditions that permit their extensive and rapid amplification should facilitate analysis of the molecular mechanisms underlying these processes and their exploitation for a variety of therapeutic applications.