STEMdiff™ T Cell Kit
STEMdiff™ Hematopoietic - EB reagents are animal component-free and optimized for lymphoid differentiation potential. Using these reagents, CD34⁺ hematopoietic progenitor cells are first generated from PSCs; these can then be differentiated to T cells using the StemSpan™ components.
⦁ Uniform generation of embryoid bodies
⦁ Robust generation of CD4+CD8+ double-positive T cells across multiple ES and iPS cell lines
- STEMdiff™ Hematopoietic - EB Basal Medium, 120 mL
- STEMdiff™ Hematopoietic - EB Supplement A, 265 μL
- STEMdiff™ Hematopoietic - EB Supplement B, 7 mL
- StemSpan™ SFEM II, 2 x 100 mL (Catalog #09605)
- StemSpan™ Lymphoid Progenitor Expansion Supplement (10X), 5 mL (Catalog #09915)
- StemSpan™ Lymphoid Differentiation Coating Material (100X), 2 x 0.25 mL (Catalog #09925)
- StemSpan™ T Cell Progenitor Maturation Supplement (10X), 12.5 mL (Catalog #09930)
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Manual | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 1 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 2 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 3 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 4 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 5 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 6 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
| Safety Data Sheet 7 | STEMdiff™ T Cell Kit | 100-0194 | All | English |
Data
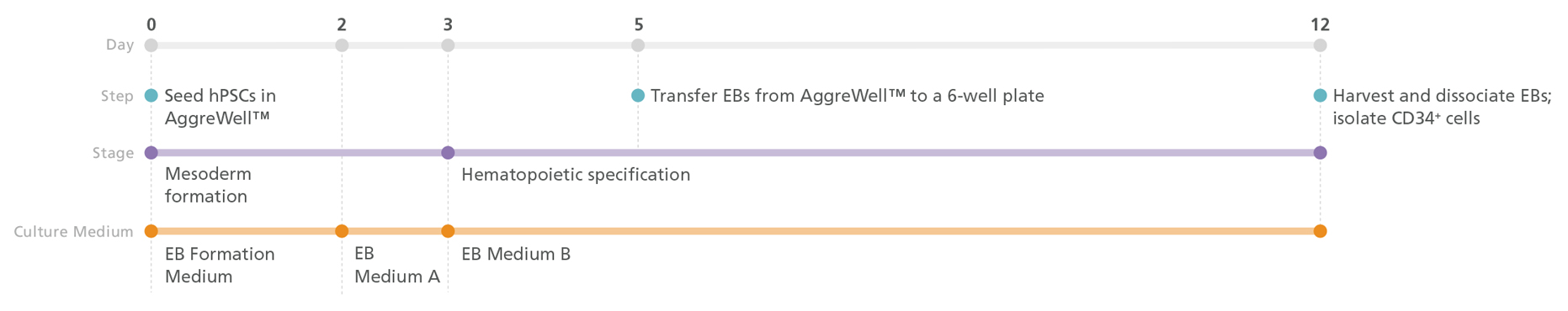
Figure 1. STEMdiff™ Hematopoietic- EB Progenitor Differentiation Protocol
PSCs are harvested and dissociated into a single-cell suspension prior to seeding into AggreWell™ plates in EB Formation Medium (EB Medium A +10 μM Y-27632) to form 500 cell aggregates. After 3 days of mesoderm formation, the medium is changed to EB Medium B to induce hematopoietic lineage differentiation. On day 5, embryoid bodies (EBs) are transferred onto non-tissue culture-treated plates. After a total of 12 days, the EBs are harvested and dissociated, then CD34+ cells are enriched by EasySep™ positive selection.
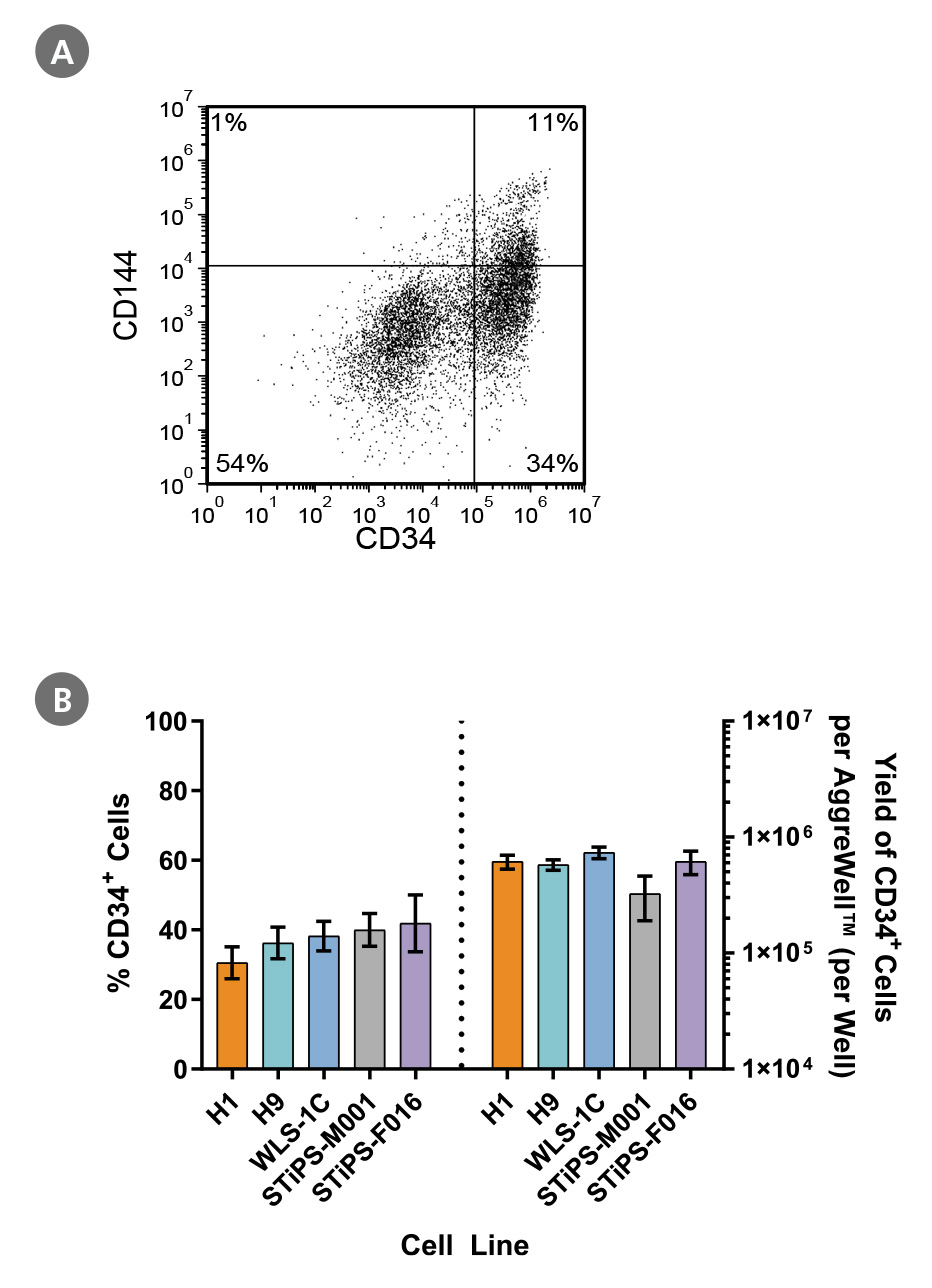
Figure 2. PSCs Differentiate to CD34+ Hematopoietic Progenitor Cells After 12 Days of Culture
Human ES and iPS cells were induced to differentiate to CD34+ cells using the 12-day protocol shown in Figure 1. At the end of the culture period, cells were harvested, dissociated into a single-cell suspension, and analyzed by flow cytometry for CD34 and CD144 expression. Dead cells were excluded by light scatter profile and DRAQ7™ staining. (A) Representative flow cytometry plot for ES (H1)-derived cells analyzed on day 12. (B) The average frequency of viable CD34+ cells on day 12 for two ES cell lines (H1 and H9) and three iPS cell lines (WLS-1C, STiPS-M001, and STiPS-F016) ranged between 31% and 42%. The average yield of CD34+ cells produced per well of a 6-well AggreWell™400 plate ranged between 3.3 x 10^5 and 7.3 x 10^5. Data are shown as mean ± SEM (n = 7 - 22).
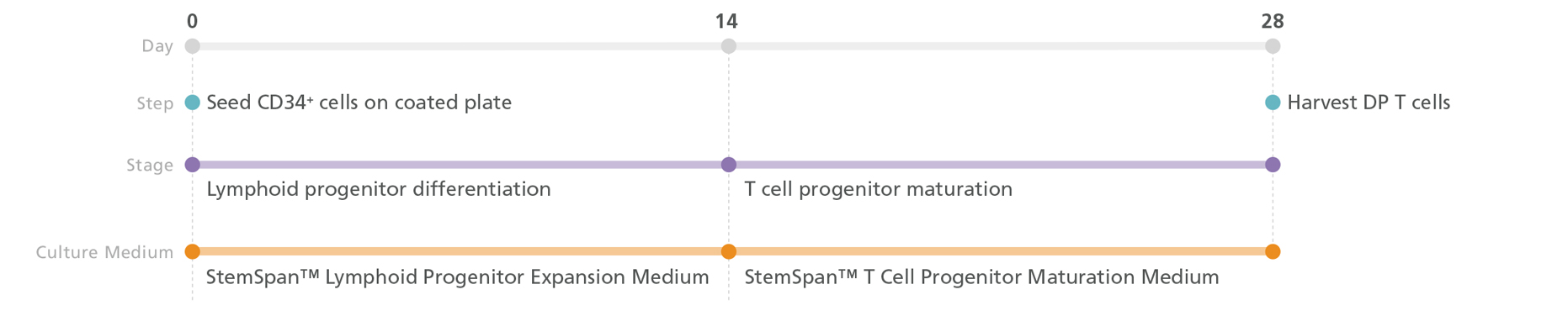
Figure 3. T Cell Generation Protocol
PSC-derived CD34+ cells are seeded in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded in StemSpan™ T Cell Progenitor Maturation Medium for further differentiation into CD4+CD8+ double-positive (DP) T cells. The DP T cells are harvested after 28 days.
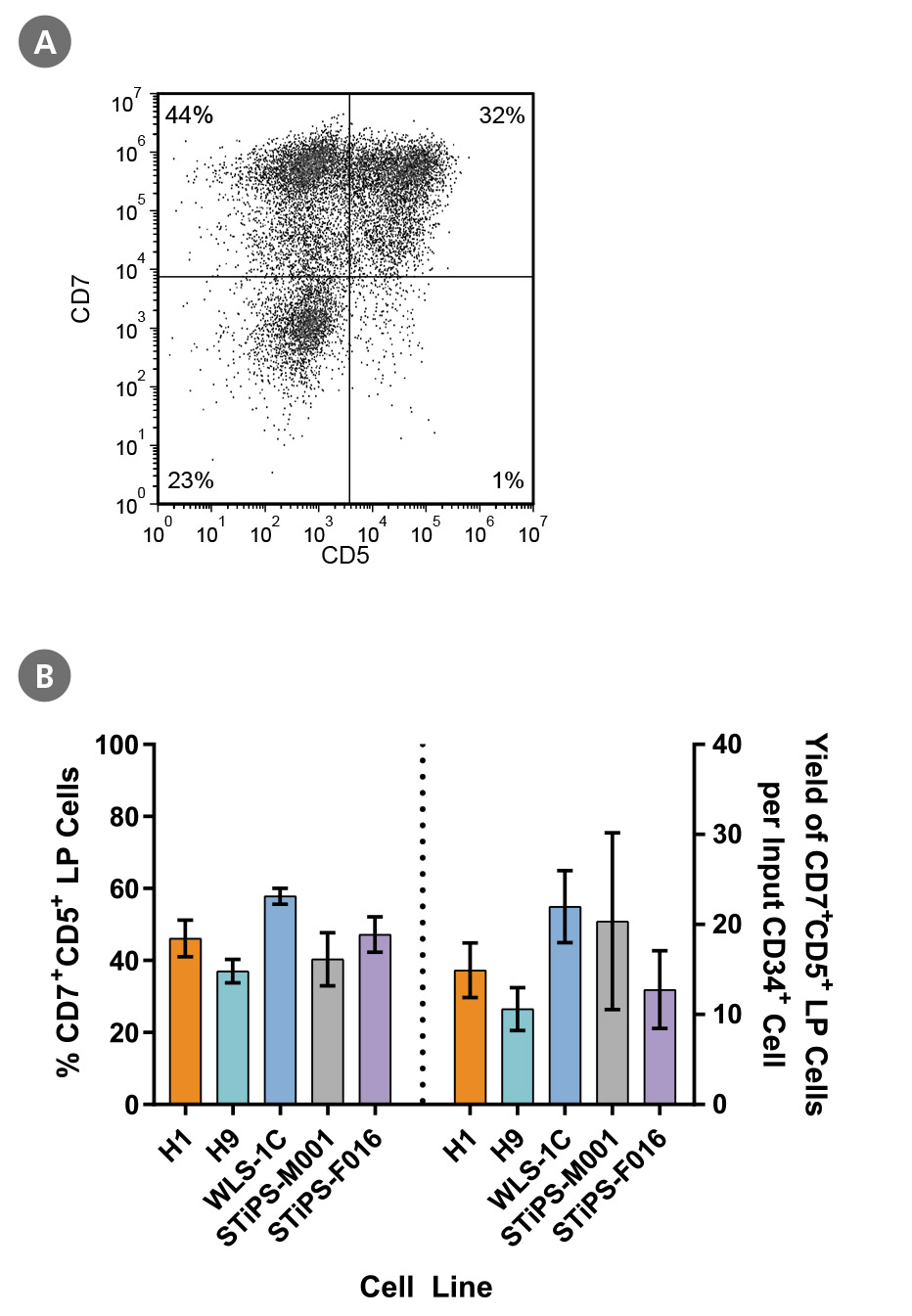
Figure 4. PSC-Derived CD34+ Cells Differentiate to CD5+CD7+ Lymphoid Progenitor Cells Over 14 Days of Culture
PSC-derived CD34+ cells were cultured for 14 days in StemSpan™ SFEM II + StemSpan™ Lymphoid Progenitor Expansion Supplement on plates coated with StemSpan™ Lymphoid Differentiation Coating Material (Figure 3). Cells were harvested and analyzed for CD7 and CD5 expression by flow cytometry. (A) Representative flow cytometry plot for ES (H1)-derived cells. (B) The average frequency of viable CD7+CD5+ lymphoid progenitor cells on day 14 ranged between 40% and 58% and the average yield of lymphoid progenitor cells produced per input PSC-derived CD34+ cell was between 11 and 22. Data are shown as mean ± SEM (n = 5 - 21).
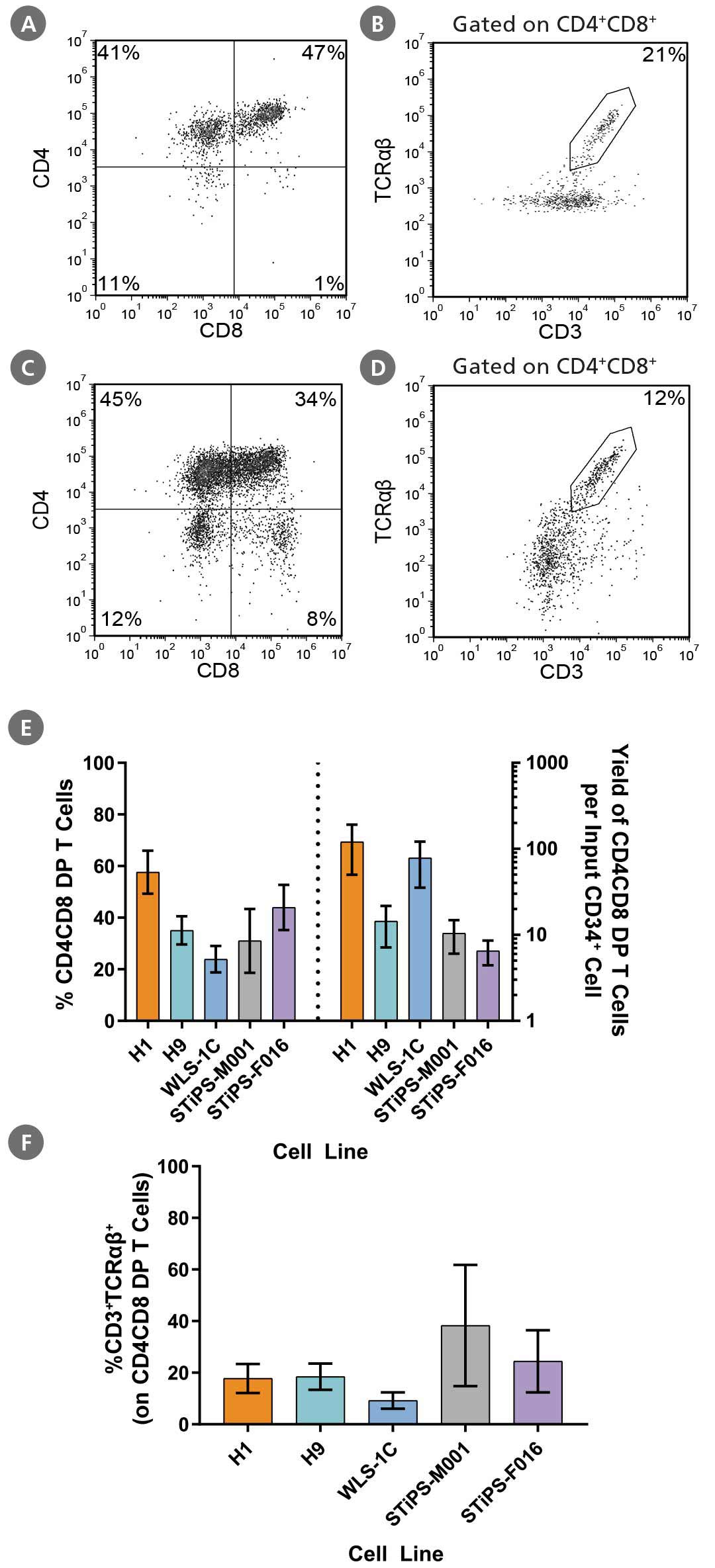
Figure 5. Generation of CD4CD8 DP T Cells From Human PSC-Derived CD34+ Cells After 28 Days of Culture
DP T cells were differentiated from PSC-derived CD34+ cells as described (Figure 3). Cells were harvested and analyzed for expression of CD3, CD4, CD8, and TCRαꞵ by flow cytometry. Representative flow cytometry plots are shown for (A, B) ES (H1)-derived and (C, D) iPS (WLS-1C)-derived cells. (E) The average frequency of viable CD4CD8 DP T cells on day 28 ranged between 24% and 58%, and the average yield of DP T cells produced per input PSC-derived CD34+ cell was between 7 and 120. (F) The average frequency of CD3+TCRαꞵ+ expressed on DP T cells ranged between 9% and 38%. Data are shown as mean ± SEM (n = 3 - 13).
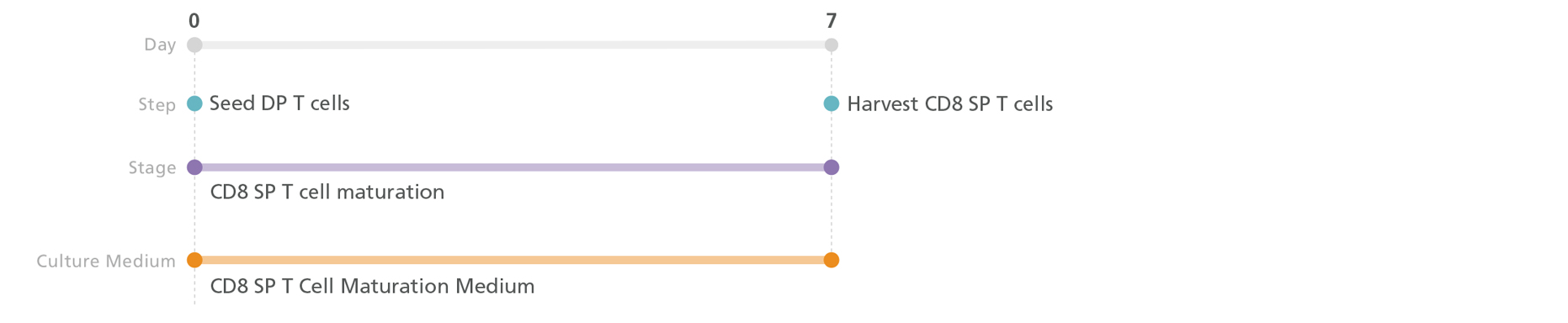
Figure 6. Optional CD8+ single-positive (SP) T Cell Maturation Protocol
DP T cells are seeded in CD8 SP T Cell Maturation Medium with added ImmunoCult™ T Cell Activator on plates coated with StemSpan™ Lymphoid Differentiation Coating Material. CD8 SP T cells can be harvested on day 7.
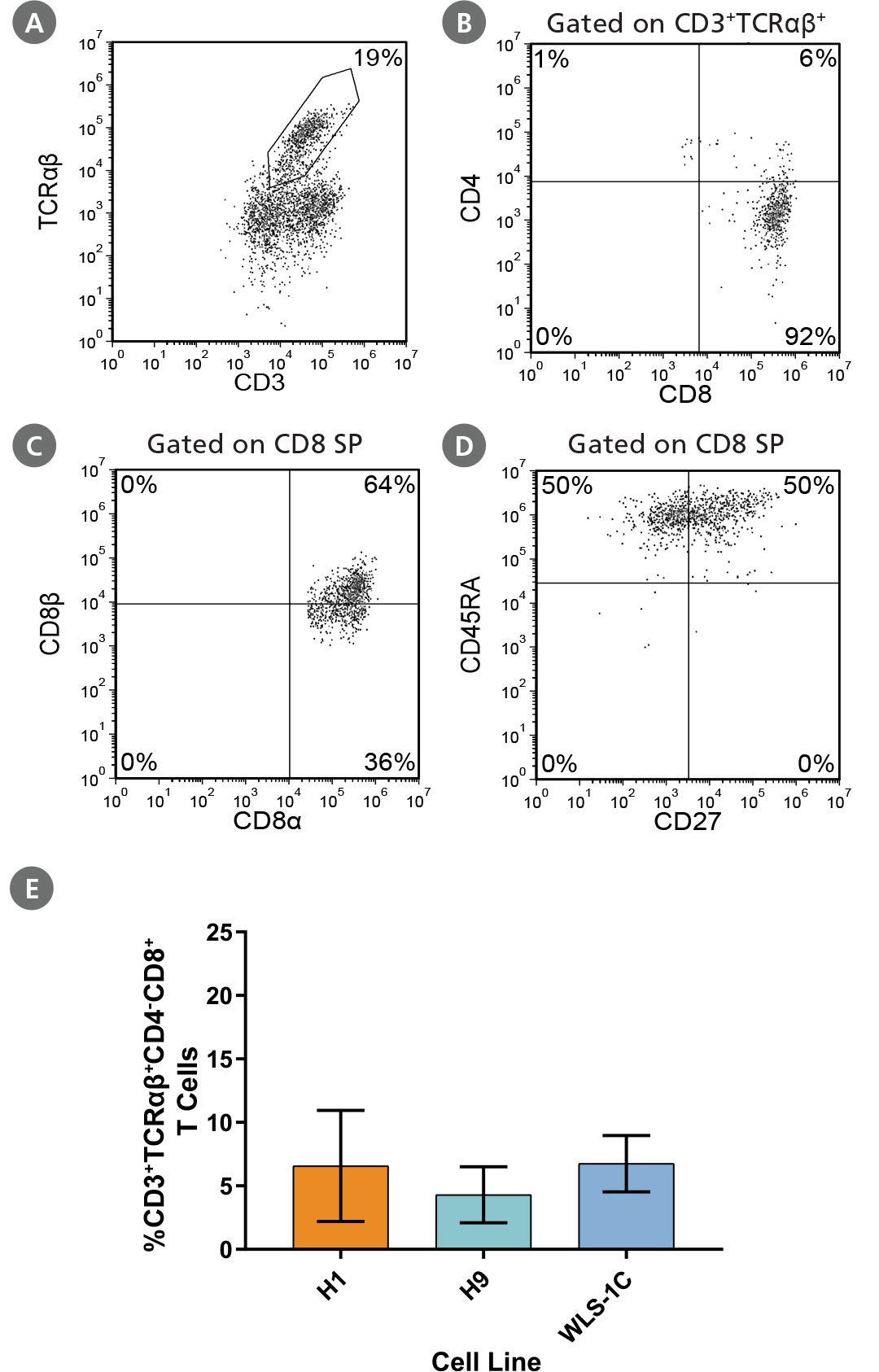
Figure 7. PSC-Derived CD4CD8 DP T Cells Are Able to Mature to CD8 SP T Cells
PSC-derived CD34+ cells were first differentiated to DP T cells during 28 days of culture and then matured to CD8 SP T cells using an additional 7-day maturation protocol (Figure 6). Cells were harvested and analyzed by flow cytometry for expression of (A) CD3 and TCRαꞵ, (B) CD4 and CD8 (gated on CD3+TCRαꞵ+), (C) CD8α and CD8β (gated on CD8 SP), and (D) CD45RA and CD27 (gated on CD8 SP). Representative results for ES (H1) cells are shown. (E) The average frequency of CD3+TCRαꞵ+CD4-CD8+ (CD8 SP) T cells in 3 cell lines was between 4% and 7%. Data are shown as mean ± SEM (n = 3 - 4).