STEMdiff™ SMADi Neural Induction Kit
Serum-free medium kit for highly efficient SMAD inhibition-mediated neural induction of human ES and iPS cells
概要
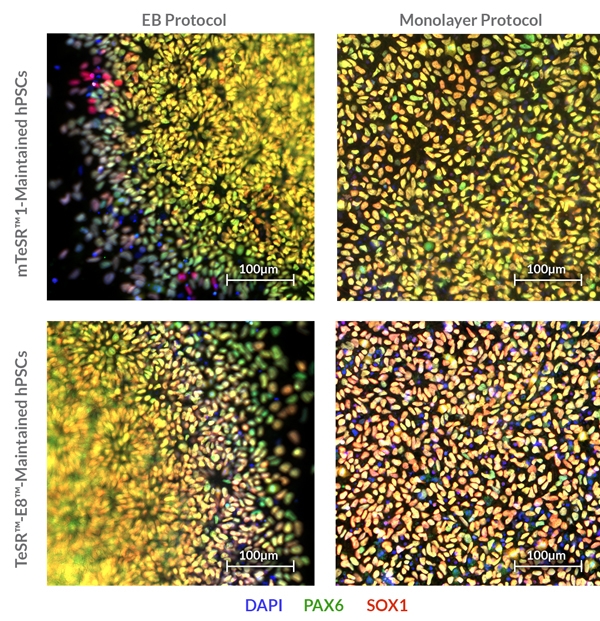
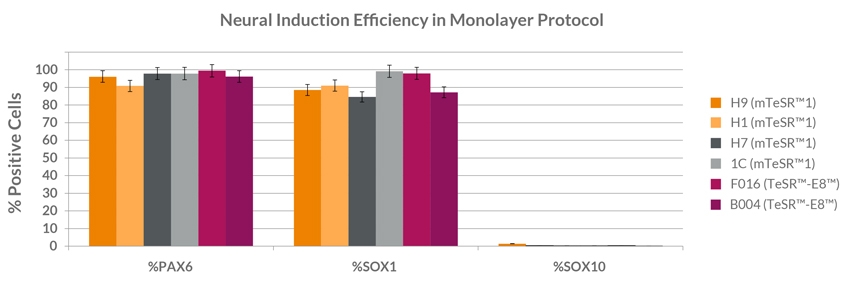
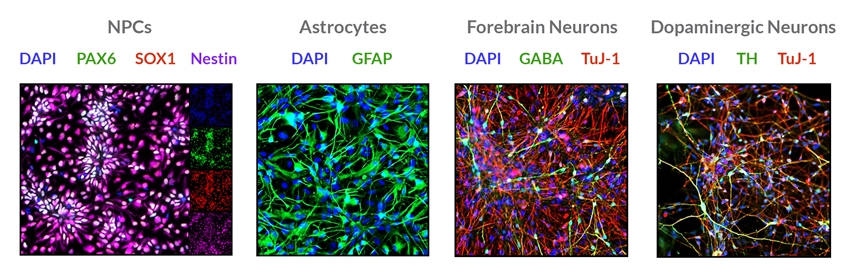
STEMdiff™ SMADi Neural Induction Kit consists of a defined, serum-free medium and supplement for the highly efficient neural induction of human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells. This kit combines STEMdiff™ Neural Induction Medium (Catalog #05835) with STEMdiff™ SMADi Neural Induction Supplement, which directs differentiation by blocking TGF-β/BMP-dependent SMAD signaling, resulting in efficient neural induction of even hard-to-differentiate cell lines. Neural progenitor cells (NPCs) can be generated using STEMdiff™ SMADi Neural Induction Kit with either an embryoid body (EB) protocol or monolayer culture protocol. The resulting cultures are enriched for central nervous system (CNS)-type NPCs, which express SOX1, Nestin, and PAX6. NPCs generated using this kit can be passaged as single cells and expanded in STEMdiff™ Neural Progenitor Medium (Catalog #05833). The NPCs can also be differentiated into neurons and glia.
• Defined and serum-free
• Promotes efficient conversion of ES and iPS cells to CNS-type NPCs, and inhibits unwanted differentiation of non-CNS cell types
• Highly efficient neural induction of even hard-to-differentiate ES and iPS cell lines
• Improves efficiency of downstream differentiation into neurons and glia
• Compatible with both embryoid body and monolayer culture protocols for neural induction
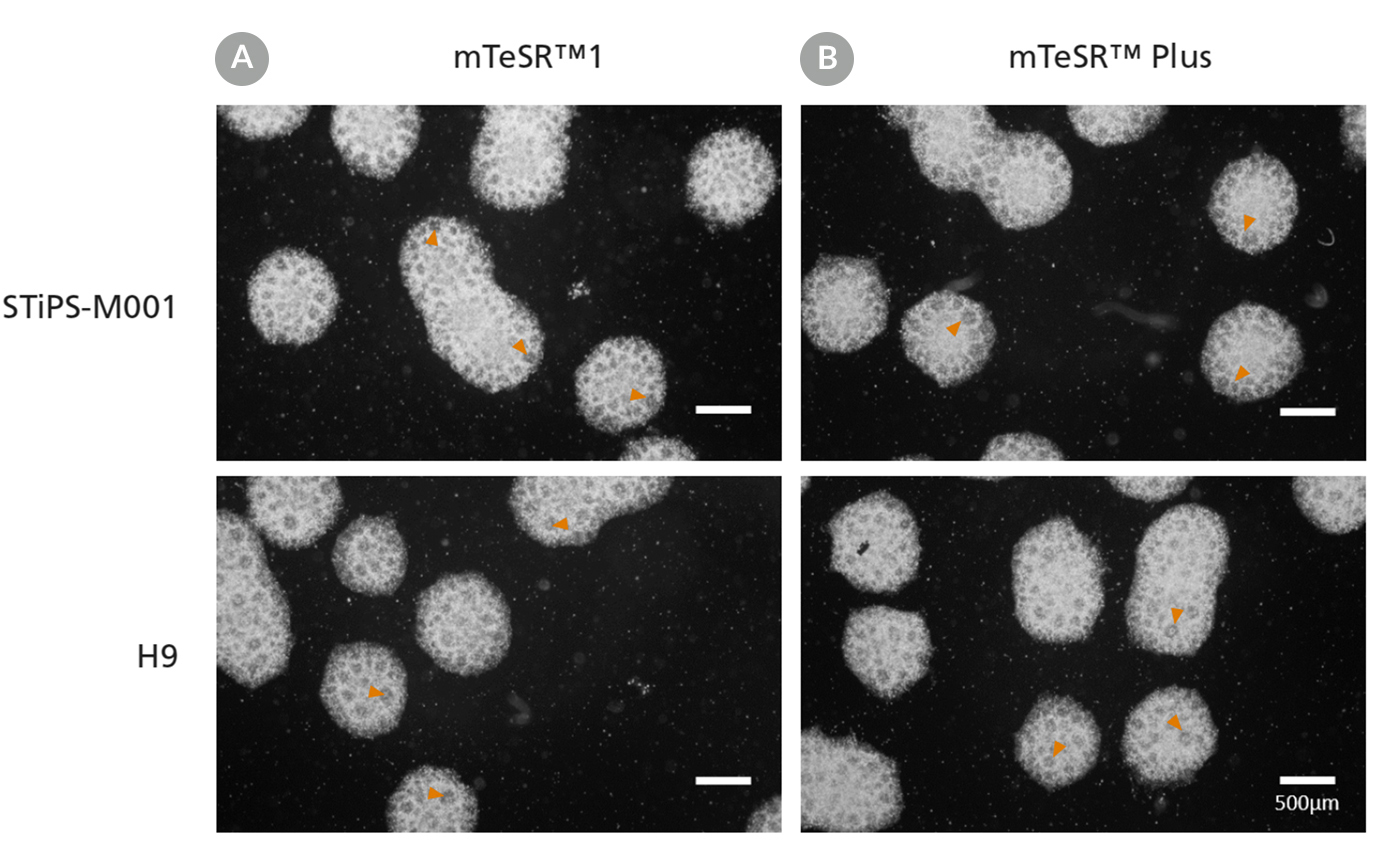
• Enables reproducible differentiation of cell lines maintained in any TeSR™ family maintenance medium
• Convenient, user-friendly format and protocols
STEMdiff™ SMADi Neural Induction Kit (Catalog #08581)
STEMdiff™ Neural Induction Medium, 250 mL
STEMdiff™ SMADi Neural Induction Supplement, 0.5 mL
STEMdiff™ SMADi Neural Induction Kit, 2 Pack (Catalog #08582)
STEMdiff™ Neural Induction Medium, 2 x 250 mL
STEMdiff™ SMADi Neural Induction Supplement, 2 x 0.5 mL
Neural Cells, PSC-Derived, Pluripotent Stem Cells
Disease Modeling, Drug Discovery and Toxicity Testing, Neuroscience, Stem Cell Biology
数据及文献
Publications (11)
Stem Cell Reports 2017 MAR
Reversal of Phenotypic Abnormalities by CRISPR/Cas9-Mediated Gene Correction in Huntington Disease Patient-Derived Induced Pluripotent Stem Cells
Xu X et al.
Abstract
Huntington disease (HD) is a dominant neurodegenerative disorder caused by a CAG repeat expansion in HTT. Here we report correction of HD human induced pluripotent stem cells (hiPSCs) using a CRISPR-Cas9 and piggyBac transposon-based approach. We show that both HD and corrected isogenic hiPSCs can be differentiated into excitable, synaptically active forebrain neurons. We further demonstrate that phenotypic abnormalities in HD hiPSC-derived neural cells, including impaired neural rosette formation, increased susceptibility to growth factor withdrawal, and deficits in mitochondrial respiration, are rescued in isogenic controls. Importantly, using genome-wide expression analysis, we show that a number of apparent gene expression differences detected between HD and non-related healthy control lines are absent between HD and corrected lines, suggesting that these differences are likely related to genetic background rather than HD-specific effects. Our study demonstrates correction of HD hiPSCs and associated phenotypic abnormalities, and the importance of isogenic controls for disease modeling using hiPSCs.
Stem Cell Reports 2017 MAR
EPHRIN-B1 Mosaicism Drives Cell Segregation in Craniofrontonasal Syndrome hiPSC-Derived Neuroepithelial Cells
Niethamer TK et al.
Abstract
Although human induced pluripotent stem cells (hiPSCs) hold great potential for the study of human diseases affecting disparate cell types, they have been underutilized in seeking mechanistic insights into the pathogenesis of congenital craniofacial disorders. Craniofrontonasal syndrome (CFNS) is a rare X-linked disorder caused by mutations in EFNB1 and characterized by craniofacial, skeletal, and neurological anomalies. Heterozygous females are more severely affected than hemizygous males, a phenomenon termed cellular interference that involves mosaicism for EPHRIN-B1 function. Although the mechanistic basis for cellular interference in CFNS has been hypothesized to involve Eph/ephrin-mediated cell segregation, no direct evidence for this has been demonstrated. Here, by generating hiPSCs from CFNS patients, we demonstrate that mosaicism for EPHRIN-B1 expression induced by random X inactivation in heterozygous females results in robust cell segregation in human neuroepithelial cells, thus supplying experimental evidence that Eph/ephrin-mediated cell segregation is relevant to pathogenesis in human CFNS patients.
Cell stem cell 2017 JAN
Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids.
E. Gabriel et al.
Abstract
The recent Zika virus (ZIKV) epidemic is associated with microcephaly in newborns. Although the connection between ZIKV and neurodevelopmental defects is widely recognized, the underlying mechanisms are poorly understood. Here we show that two recently isolated strains of ZIKV, an American strain from an infected fetal brain (FB-GWUH-2016) and a closely-related Asian strain (H/PF/2013), productively infect human iPSC-derived brain organoids. Both of these strains readily target to and replicate in proliferating ventricular zone (VZ) apical progenitors. The main phenotypic effect was premature differentiation of neural progenitors associated with centrosome perturbation, even during early stages of infection, leading to progenitor depletion, disruption of the VZ, impaired neurogenesis, and cortical thinning. The infection pattern and cellular outcome differ from those seen with the extensively passaged ZIKV strain MR766. The structural changes we see after infection with these more recently isolated viral strains closely resemble those seen in ZIKV-associated microcephaly.
Science Translational Medicine 2017 FEB
Tumor-homing cytotoxic human induced neural stem cells for cancer therapy
Bagó et al.
Abstract
Engineered neural stem cells (NSCs) are a promising approach to treating glioblastoma (GBM). The ideal NSC drug carrier for clinical use should be easily isolated and autologous to avoid immune rejection. We transdifferentiated (TD) human fibroblasts into tumor-homing early-stage induced NSCs (h-iNSC(TE)), engineered them to express optical reporters and different therapeutic gene products, and assessed the tumor-homing migration and therapeutic efficacy of cytotoxic h-iNSC(TE) in patient-derived GBM models of surgical and nonsurgical disease. Molecular and functional analysis revealed that our single-factor SOX2 TD strategy converted human skin fibroblasts into h-iNSC(TE) that were nestin(+) and expressed pathways associated with tumor-homing migration in 4 days. Time-lapse motion analysis showed that h-iNSC(TE) rapidly migrated to human GBM cells and penetrated human GBM spheroids, a process inhibited by blockade of CXCR4. Serial imaging showed that h-iNSC(TE) delivery of the proapoptotic agent tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) reduced the size of solid human GBM xenografts 250-fold in 3 weeks and prolonged median survival from 22 to 49 days. Additionally, h-iNSC(TE) thymidine kinase/ganciclovir enzyme/prodrug therapy (h-iNSC(TE)-TK) reduced the size of patient-derived GBM xenografts 20-fold and extended survival from 32 to 62 days. Mimicking clinical NSC therapy, h-iNSC(TE)-TK therapy delivered into the postoperative surgical resection cavity delayed the regrowth of residual GBMs threefold and prolonged survival from 46 to 60 days. These results suggest that TD of human skin into h-iNSC(TE) is a platform for creating tumor-homing cytotoxic cell therapies for cancer, where the potential to avoid carrier rejection could maximize treatment durability in human trials.
Stem cell reports 2016 JAN
Development and Dynamic Regulation of Mitochondrial Network in Human Midbrain Dopaminergic Neurons Differentiated from iPSCs.
E. Gabriel et al.
Abstract
Mitochondria are critical to neurogenesis, but the mechanisms of mitochondria in neurogenesis have not been well explored. We fully characterized mitochondrial alterations and function in relation to the development of human induced pluripotent stem cell (hiPSC)-derived dopaminergic (DA) neurons. Following directed differentiation of hiPSCs to DA neurons, mitochondria in these neurons exhibit pronounced changes during differentiation, including mature neurophysiology characterization and functional synaptic network formation. Inhibition of mitochondrial respiratory chains via application of complex IV inhibitor KCN (potassium cyanide) or complex I inhibitor rotenone restricted neurogenesis of DA neurons. These results demonstrated the direct importance of mitochondrial development and bioenergetics in DA neuronal differentiation. Our study also provides a neurophysiologic model of mitochondrial involvement in neurogenesis, which will enhance our understanding of the role of mitochondrial dysfunctions in neurodegenerative diseases.
Glia 2016 JAN
Generation of GFAP::GFP astrocyte reporter lines from human adult fibroblast-derived iPS cells using zinc-finger nuclease technology.
Zhang P-WW et al.
Abstract
Astrocytes are instrumental to major brain functions, including metabolic support, extracellular ion regulation, the shaping of excitatory signaling events and maintenance of synaptic glutamate homeostasis. Astrocyte dysfunction contributes to numerous developmental, psychiatric and neurodegenerative disorders. The generation of adult human fibroblast-derived induced pluripotent stem cells (iPSCs) has provided novel opportunities to study mechanisms of astrocyte dysfunction in human-derived cells. To overcome the difficulties of cell type heterogeneity during the differentiation process from iPSCs to astroglial cells (iPS astrocytes), we generated homogenous populations of iPS astrocytes using zinc-finger nuclease (ZFN) technology. Enhanced green fluorescent protein (eGFP) driven by the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter was inserted into the safe harbor adeno-associated virus integration site 1 (AAVS1) locus in disease and control-derived iPSCs. Astrocyte populations were enriched using Fluorescence Activated Cell Sorting (FACS) and after enrichment more than 99% of iPS astrocytes expressed mature astrocyte markers including GFAP, S100$\$, NFIA and ALDH1L1. In addition, mature pure GFP-iPS astrocytes exhibited a well-described functional astrocytic activity in vitro characterized by neuron-dependent regulation of glutamate transporters to regulate extracellular glutamate concentrations. Engraftment of GFP-iPS astrocytes into rat spinal cord grey matter confirmed in vivo cell survival and continued astrocytic maturation. In conclusion, the generation of GFAP::GFP-iPS astrocytes provides a powerful in vitro and in vivo tool for studying astrocyte biology and astrocyte-driven disease pathogenesis and therapy.
View All Publications