STEMdiff™ Definitive Endoderm Kit
Defined animal component-free medium for the differentiation of human ES and iPS cells to definitive endoderm
概要
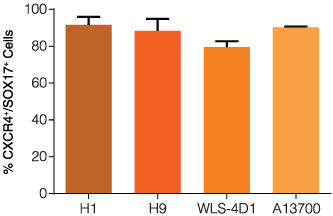
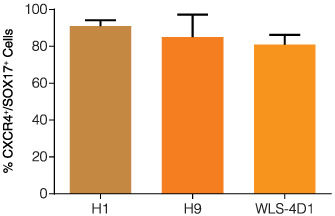
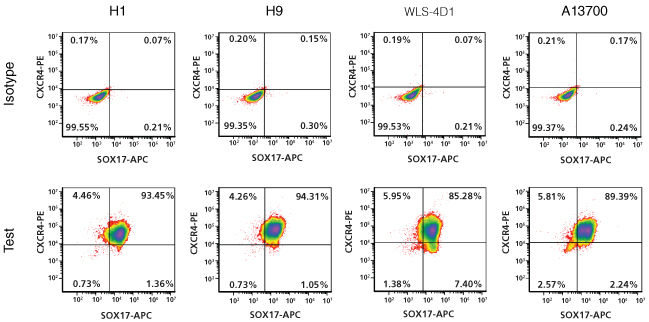
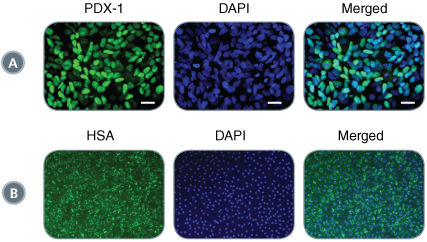
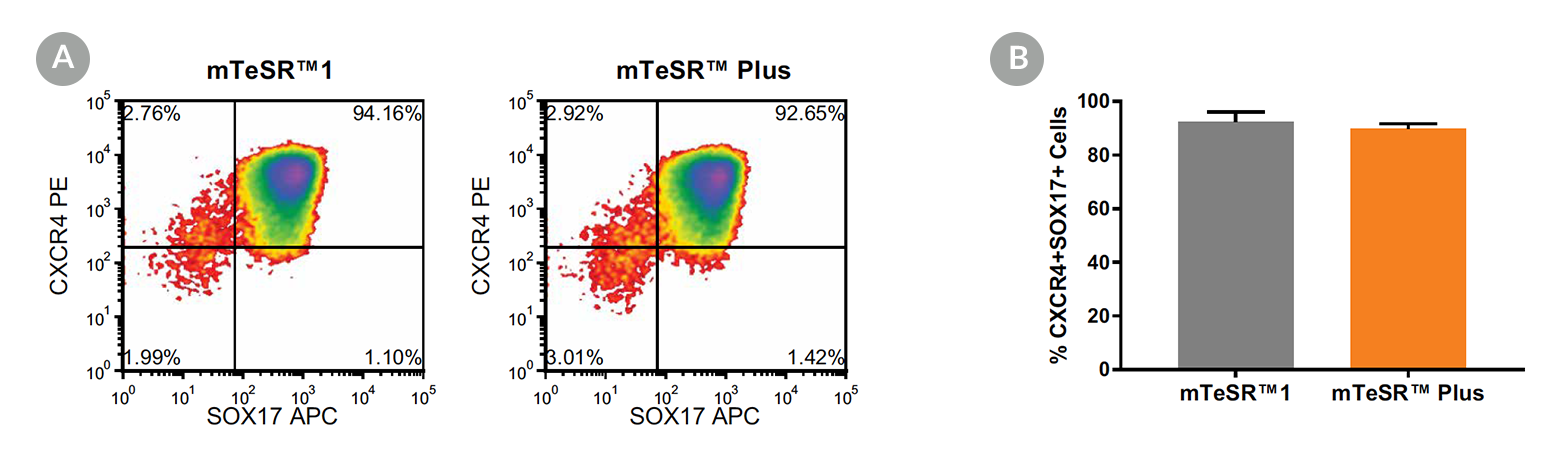
STEMdiff™ Definitive Endoderm Kit is a complete, serum- and animal component-free medium and supplement kit that supports highly efficient differentiation of human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to definitive endoderm cells. Cells differentiated using STEMdiff™ Definitive Endoderm Kit express high levels of endoderm markers, including CD184 (CXCR4), SOX17, FOXA2, and c-KIT, and lack expression of ectoderm, mesoderm, and pluripotency markers. The definitive endoderm cells produced using this kit are multipotent and capable of further differentiation towards cells of the pancreatic, intestinal, pulmonary, and hepatic lineages, thus providing a robust tool for developmental studies, disease modeling, and drug discovery.
This kit is optimized for differentiation of cells maintained in mTeSR™1. For differentiation of cells maintained in TeSR™-E8™, please see the
STEMDiff™ Definitive Endoderm Kit (TeSR™-E8™ Optimized).
• Defined, serum-free, animal component-free medium for the differentiation of human ES and iPS cells to definitive endoderm in a complete, ready-to-use format
• Efficient and reproducible differentiation of multiple ES cell and iPS cell lines
• Generates definitive endoderm cells capable of further differentiation to pancreatic, hepatic, intestinal and pulmonary cell lineages
• Optimized for use with cells maintained in TeSR™-E8™ cell culture medium
- STEMdiff™ Endoderm Basal Medium, 100 mL
- STEMdiff™ Definitive Endoderm Supplement MR (100X), 0.35 mL
- STEMdiff™ Definitive Endoderm Supplement CJ (100X), 1.1 mL
Airway Cells, Endoderm, PSC-Derived, Hepatic Cells, Intestinal Cells, Pancreatic Cells, Pluripotent Stem Cells
Cell Culture, Differentiation
Cancer Research, Epithelial Cell Biology, Stem Cell Biology
数据及文献
Publications (21)
Translational psychiatry 2019
Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism.
L.-M. Grunwald et al.
Abstract
Human induced pluripotent stem cells (hiPSC) provide an attractive tool to study disease mechanisms of neurodevelopmental disorders such as schizophrenia. A pertinent problem is the development of hiPSC-based assays to discriminate schizophrenia (SZ) from autism spectrum disorder (ASD) models. Healthy control individuals as well as patients with SZ and ASD were examined by a panel of diagnostic tests. Subsequently, skin biopsies were taken for the generation, differentiation, and testing of hiPSC-derived neurons from all individuals. SZ and ASD neurons share a reduced capacity for cortical differentiation as shown by quantitative analysis of the synaptic marker PSD95 and neurite outgrowth. By contrast, pattern analysis of calcium signals turned out to discriminate among healthy control, schizophrenia, and autism samples. Schizophrenia neurons displayed decreased peak frequency accompanied by increased peak areas, while autism neurons showed a slight decrease in peak amplitudes. For further analysis of the schizophrenia phenotype, transcriptome analyses revealed a clear discrimination among schizophrenia, autism, and healthy controls based on differentially expressed genes. However, considerable differences were still evident among schizophrenia patients under inspection. For one individual with schizophrenia, expression analysis revealed deregulation of genes associated with the major histocompatibility complex class II (MHC class II) presentation pathway. Interestingly, antipsychotic treatment of healthy control neurons also increased MHC class II expression. In conclusion, transcriptome analysis combined with pattern analysis of calcium signals appeared as a tool to discriminate between SZ and ASD phenotypes in vitro.
Cell 2018 JAN
Intrinsic Immunity Shapes Viral Resistance of Stem Cells.
Wu X et al.
Abstract
Stem cells are highly resistant to viral infection compared to their differentiated progeny; however, the mechanism is mysterious. Here, we analyzed gene expression in mammalian stem cells and cells at various stages of differentiation. We find that, conserved across species, stem cells express a subset of genes previously classified as interferon (IFN) stimulated genes (ISGs) but that expression is intrinsic, as stem cells are refractory to interferon. This intrinsic ISG expression varies in a cell-type-specific manner, and many ISGs decrease upon differentiation, at which time cells become IFN responsive, allowing induction of a broad spectrum of ISGs by IFN signaling. Importantly, we show that intrinsically expressed ISGs protect stem cells against viral infection. We demonstrate the in vivo importance of intrinsic ISG expression for protecting stem cells and their differentiation potential during viral infection. These findings have intriguing implications for understanding stem cell biology and the evolution of pathogen resistance.
Stem Cell Research 2016 MAR
Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen
Carpentier A et al.
Abstract
The establishment of protocols to differentiate human pluripotent stem cells (hPSCs) including embryonic (ESC) and induced pluripotent (iPSC) stem cells into functional hepatocyte-like cells (HLCs) creates new opportunities to study liver metabolism, genetic diseases and infection of hepatotropic viruses (hepatitis B and C viruses) in the context of specific genetic background. While supporting efficient differentiation to HLCs, the published protocols are limited in terms of differentiation into fully mature hepatocytes and in a smaller-well format. This limitation handicaps the application of these cells to high-throughput assays. Here we describe a protocol allowing efficient and consistent hepatic differentiation of hPSCs in 384-well plates into functional hepatocyte-like cells, which remain differentiated for more than 3 weeks. This protocol affords the unique opportunity to miniaturize the hPSC-based differentiation technology and facilitates screening for molecules in modulating liver differentiation, metabolism, genetic network, and response to infection or other external stimuli.
Stem Cell Research 2016 MAR
The Forkhead box transcription factor FOXM1 is required for the maintenance of cell proliferation and protection against oxidative stress in human embryonic stem cells
Kwok CTD et al.
Abstract
Human embryonic stem cells (hESCs) exhibit unique cell cycle structure, self-renewal and pluripotency. The Forkhead box transcription factor M1 (FOXM1) is critically required for the maintenance of pluripotency in mouse embryonic stem cells and mouse embryonal carcinoma cells, but its role in hESCs remains unclear. Here, we show that FOXM1 expression was enriched in undifferentiated hESCs and was regulated in a cell cycle-dependent manner with peak levels detected at the G2/M phase. Expression of FOXM1 did not correlate with OCT4 and NANOG during in vitro differentiation of hESCs. Importantly, knockdown of FOXM1 expression led to aberrant cell cycle distribution with impairment in mitotic progression but showed no profound effect on the undifferentiated state. Interestingly, FOXM1 depletion sensitized hESCs to oxidative stress. Moreover, genome-wide analysis of FOXM1 targets by ChIP-seq identified genes important for M phase including CCNB1 and CDK1, which were subsequently confirmed by ChIP and RNA interference analyses. Further peak set comparison against a differentiating hESC line and a cancer cell line revealed a substantial difference in the genomic binding profile of FOXM1 in hESCs. Taken together, our findings provide the first evidence to support FOXM1 as an important regulator of cell cycle progression and defense against oxidative stress in hESCs.
Methods in molecular biology (Clifton, N.J.) 2016 JAN
Multisystemic Disease Modeling of Liver-Derived Protein Folding Disorders Using Induced Pluripotent Stem Cells (iPSCs).
Leung A and Murphy GJ
Abstract
Familial transthyretin amyloidosis (ATTR) is an autosomal dominant protein-folding disorder caused by over 100 distinct mutations in the transthyretin (TTR) gene. In ATTR, protein secreted from the liver aggregates and forms fibrils in target organs, chiefly the heart and peripheral nervous system, highlighting the need for a model capable of recapitulating the multisystem complexity of this clinically variable disease. Here, we describe detailed methodologies for the directed differentiation of protein folding disease-specific iPSCs into hepatocytes that produce mutant protein, and neural-lineage cells often targeted in disease. Methodologies are also described for the construction of multisystem models and drug screening using iPSCs.
Tissue engineering. Part C, Methods 2016 JAN
Recombinant Xeno-Free Vitronectin Supports Self-Renewal and Pluripotency in Protein-Induced Pluripotent Stem Cells.
Kaini RR et al.
Abstract
Patient safety is a major concern in the application of induced pluripotent stem cells (iPSCs) in cell-based therapy. Efforts are being made to reprogram, maintain, and differentiate iPSCs in defined conditions to provide a safe source of stem cells for regenerative medicine. Recently, human fibroblasts were successfully reprogrammed into pluripotent stem cells using four recombinant proteins (OCT4, c-Myc, KLF4, and SOX2) fused with a cell-penetrating peptide (9R). These protein-induced pluripotent stem cells (piPSCs) are maintained and propagated on a feeder layer of mouse embryonic fibroblasts. Use of animal-derived products in maintenance and differentiation of iPSCs poses risks of zoonotic disease transmission and immune rejection when transplanted into humans. To avoid potential incorporation of xenogenic products, we cultured piPSCs on recombinant human matrix proteins. We then tested whether recombinant human matrix proteins can support self-renewal and pluripotency of piPSCs. After long-term culture on recombinant human vitronectin in xeno-free conditions, piPSCs retained the expression of pluripotent markers. The pluripotency of these cells was further evaluated by differentiating toward ectoderm, mesoderm, and endoderm lineages in vitro. In conclusion, recombinant human vitronectin can support the long-term culture and maintain the stemness of piPSCs in defined nonxenogenic conditions.
View All Publications