STEMdiff™ Cerebral Organoid Kit
Culture medium kit for establishment and maturation of human cerebral organoids
概要
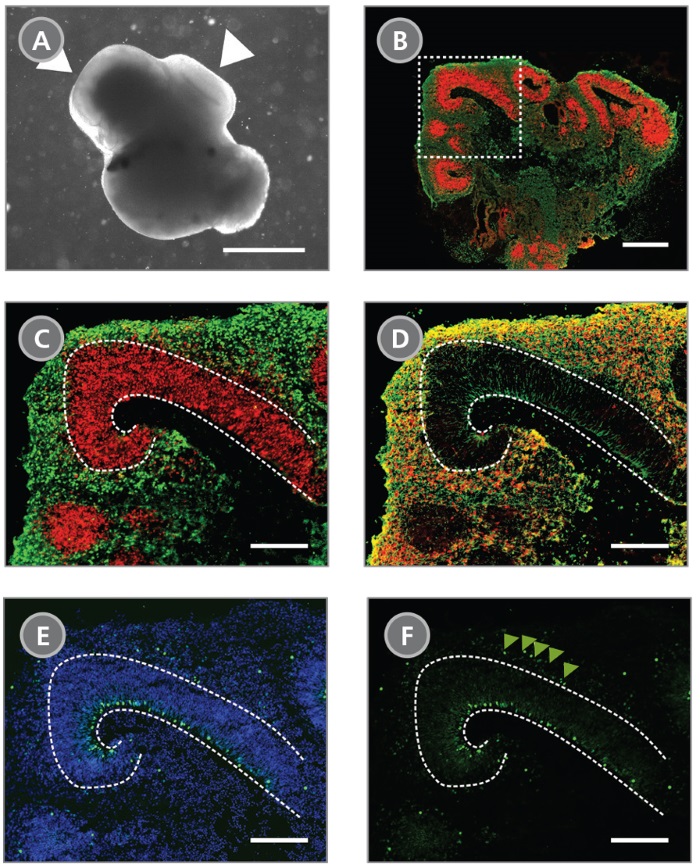
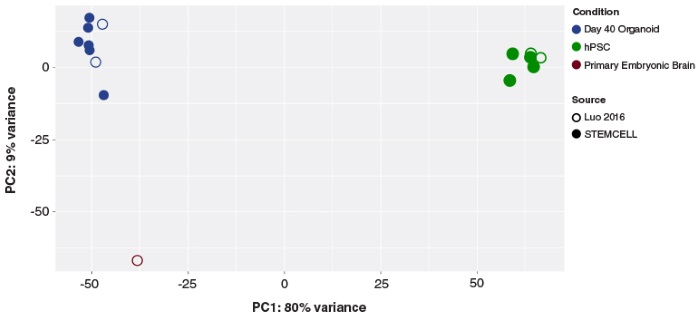
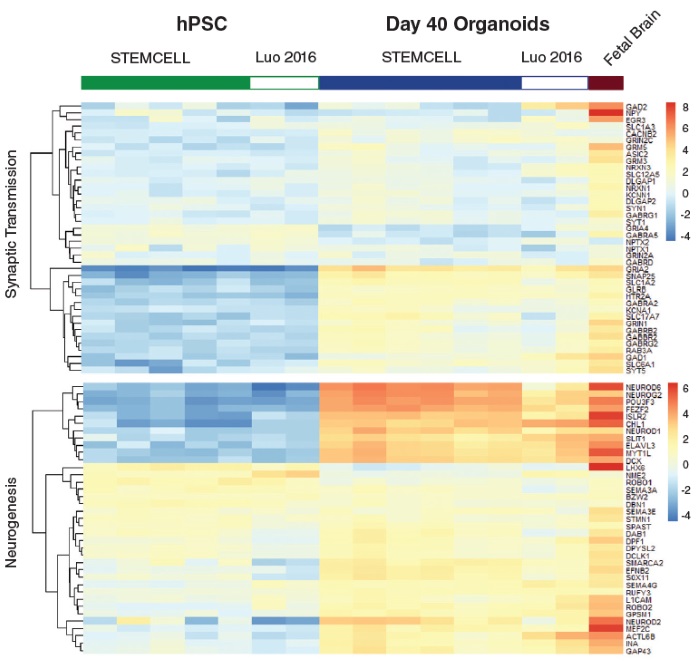
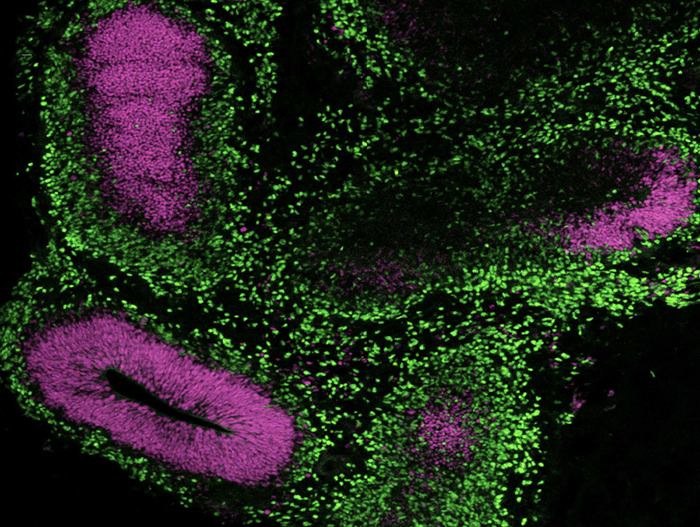
STEMdiff™ Cerebral Organoid Kit (Catalog #08570) is a defined, serum-free cell culture medium that enables the robust generation of human pluripotent stem cell (hPSC)-derived cerebral organoids in a simple four-stage protocol. Cerebral organoids are three-dimensional in vitro models with a cellular composition and structural organization that is representative of the developing human brain. STEMdiff™ Cerebral Organoid Kit has been optimized to increase efficiency and reproducibility of organoid formation based on the formulation published by Lancaster et al. (Lancaster MA et al. Nature USA, 2013 and Lancaster MA et al. Science USA, 2014). Cerebral organoid formation is initiated through an intermediate embryoid body (EB) formation step followed by expansion of neuroepithelia. After a period of maturation, organoids generated using this kit feature cortical-like regions such as the ventricular zone (PAX6+/SOX2+/Ki-67+), outer subventricular zone (Ki-67+/p-Vimentin+), intermediate zone (TBR2+), and cortical plate (CTIP2+/MAP2+/TBR1+), which layer in similar orientations as observed in vivo. For extended periods of organoid culture (> 40 days), the components required for organoid maturation can be purchased as the STEMdiff™ Cerebral Organoid Maturation Kit (Catalog #08571).
Neural Cells, PSC-Derived, Neural Stem and Progenitor Cells, Pluripotent Stem Cells
Cell Culture, Differentiation, Organoid Culture
Disease Modeling, Neuroscience, Stem Cell Biology
数据及文献
Publications (8)
Science (New York, N.Y.) 2020
Human CNS barrier-forming organoids with cerebrospinal fluid production.
L. Pellegrini et al.
Abstract
Cerebrospinal fluid (CSF) is a vital liquid, providing nutrients and signaling molecules and clearing out toxic by-products from the brain. The CSF is produced by the choroid plexus (ChP), a protective epithelial barrier that also prevents free entry of toxic molecules or drugs from the blood. Here, we establish human ChP organoids with a selective barrier and CSF-like fluid secretion in self-contained compartments. We show that this in vitro barrier exhibits the same selectivity to small molecules as the ChP in vivo and that ChP-CSF organoids can predict central nervous system (CNS) permeability of new compounds. The transcriptomic and proteomic signatures of ChP-CSF organoids reveal a high degree of similarity to the ChP in vivo. Finally, the intersection of single-cell transcriptomics and proteomic analysis uncovers key human CSF components produced by previously unidentified specialized epithelial subtypes.
Analytical chemistry 2020
One-Stop Microfluidic Assembly of Human Brain Organoids To Model Prenatal Cannabis Exposure.
Z. Ao et al.
Abstract
Prenatal cannabis exposure (PCE) influences human brain development, but it is challenging to model PCE using animals and current cell culture techniques. Here, we developed a one-stop microfluidic platform to assemble and culture human cerebral organoids from human embryonic stem cells (hESC) to investigate the effect of PCE on early human brain development. By incorporating perfusable culture chambers, air-liquid interface, and one-stop protocol, this microfluidic platform can simplify the fabrication procedure and produce a large number of organoids (169 organoids per 3.5 cm × 3.5 cm device area) without fusion, as compared with conventional fabrication methods. These one-stop microfluidic assembled cerebral organoids not only recapitulate early human brain structure, biology, and electrophysiology but also have minimal size variation and hypoxia. Under on-chip exposure to the psychoactive cannabinoid, $\Delta$-9-tetrahydrocannabinol (THC), cerebral organoids exhibited reduced neuronal maturation, downregulation of cannabinoid receptor type 1 (CB1) receptors, and impaired neurite outgrowth. Moreover, transient on-chip THC treatment also decreased spontaneous firing in these organoids. This one-stop microfluidic technique enables a simple, scalable, and repeatable organoid culture method that can be used not only for human brain organoids but also for many other human organoids including liver, kidney, retina, and tumor organoids. This technology could be widely used in modeling brain and other organ development, developmental disorders, developmental pharmacology and toxicology, and drug screening.
Scientific reports 2019 nov
Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-$\gamma$ Responses in a Monocyte-Dependent Manner.
M. Mata Forsberg et al.
Abstract
Secreted factors derived from Lactobacillus are able to dampen pro-inflammatory cytokine responses. Still, the nature of these components and the underlying mechanisms remain elusive. Here, we aimed to identify the components and the mechanism involved in the Lactobacillus-mediated modulation of immune cell activation. PBMC were stimulated in the presence of the cell free supernatants (CFS) of cultured Lactobacillus rhamnosus GG and Lactobacillus reuteri DSM 17938, followed by evaluation of cytokine responses. We show that lactobacilli-CFS effectively dampen induced IFN-$\gamma$ and IL-17A responses from T- and NK cells in a monocyte dependent manner by a soluble factor. A proteomic array analysis highlighted Lactobacillus-induced IL-1 receptor antagonist (ra) as a potential candidate responsible for the IFN-$\gamma$ dampening activity. Indeed, addition of recombinant IL-1ra to stimulated PBMC resulted in reduced IFN-$\gamma$ production. Further characterization of the lactobacilli-CFS revealed the presence of extracellular membrane vesicles with a similar immune regulatory activity to that observed with the lactobacilli-CFS. In conclusion, we have shown that lactobacilli produce extracellular MVs, which are able to dampen pro-inflammatory cytokine responses in a monocyte-dependent manner.
Cell stem cell 2019 aug
Interconversion between Tumorigenic and Differentiated States in Acute Myeloid Leukemia.
M. D. McKenzie et al.
Abstract
Tumors are composed of phenotypically heterogeneous cancer cells that often resemble various differentiation states of their lineage of origin. Within this hierarchy, it is thought that an immature subpopulation of tumor-propagating cancer stem cells (CSCs) differentiates into non-tumorigenic progeny, providing a rationale for therapeutic strategies that specifically eradicate CSCs or induce their differentiation. The clinical success of these approaches depends on CSC differentiation being unidirectional rather than reversible, yet this question remains unresolved even in prototypically hierarchical malignancies, such as acute myeloid leukemia (AML). Here, we show in murine and human models of AML that, upon perturbation of endogenous expression of the lineage-determining transcription factor PU.1 or withdrawal of established differentiation therapies, some mature leukemia cells can de-differentiate and reacquire clonogenic and leukemogenic properties. Our results reveal plasticity of CSC maturation in AML, highlighting the need to therapeutically eradicate cancer cells across a range of differentiation states.
Nature Neuroscience 2019 apr
Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output
S. L. Giandomenico et al.
Abstract
Neural organoids have the potential to improve our understanding of human brain development and neurological disorders. However, it remains to be seen whether these tissues can model circuit formation with functional neuronal output. Here we have adapted air–liquid interface culture to cerebral organoids, leading to improved neuronal survival and axon outgrowth. The resulting thick axon tracts display various morphologies, including long-range projection within and away from the organoid, growth-cone turning, and decussation. Single-cell RNA sequencing reveals various cortical neuronal identities, and retrograde tracing demonstrates tract morphologies that match proper molecular identities. These cultures exhibit active neuronal networks, and subcortical projecting tracts can innervate mouse spinal cord explants and evoke contractions of adjacent muscle in a manner dependent on intact organoid-derived innervating tracts. Overall, these results reveal a remarkable self-organization of corticofugal and callosal tracts with a functional output, providing new opportunities to examine relevant aspects of human CNS development and disease.
eLife 2019
Mechanisms of hyperexcitability in Alzheimer's disease hiPSC-derived neurons and cerebral organoids vs isogenic controls.
S. Ghatak et al.
Abstract
Human Alzheimer's disease (AD) brains and transgenic AD mouse models manifest hyperexcitability. This aberrant electrical activity is caused by synaptic dysfunction that represents the major pathophysiological correlate of cognitive decline. However, the underlying mechanism for this excessive excitability remains incompletely understood. To investigate the basis for the hyperactivity, we performed electrophysiological and immunofluorescence studies on hiPSC-derived cerebrocortical neuronal cultures and cerebral organoids bearing AD-related mutations in presenilin-1 or amyloid precursor protein vs. isogenic gene corrected controls. In the AD hiPSC-derived neurons/organoids, we found increased excitatory bursting activity, which could be explained in part by a decrease in neurite length. AD hiPSC-derived neurons also displayed increased sodium current density and increased excitatory and decreased inhibitory synaptic activity. Our findings establish hiPSC-derived AD neuronal cultures and organoids as a relevant model of early AD pathophysiology and provide mechanistic insight into the observed hyperexcitability.
View All Publications