PneumaCult™-Ex Plus Medium
Serum- and BPE-free medium for expansion of primary human airway epithelial cells
概要
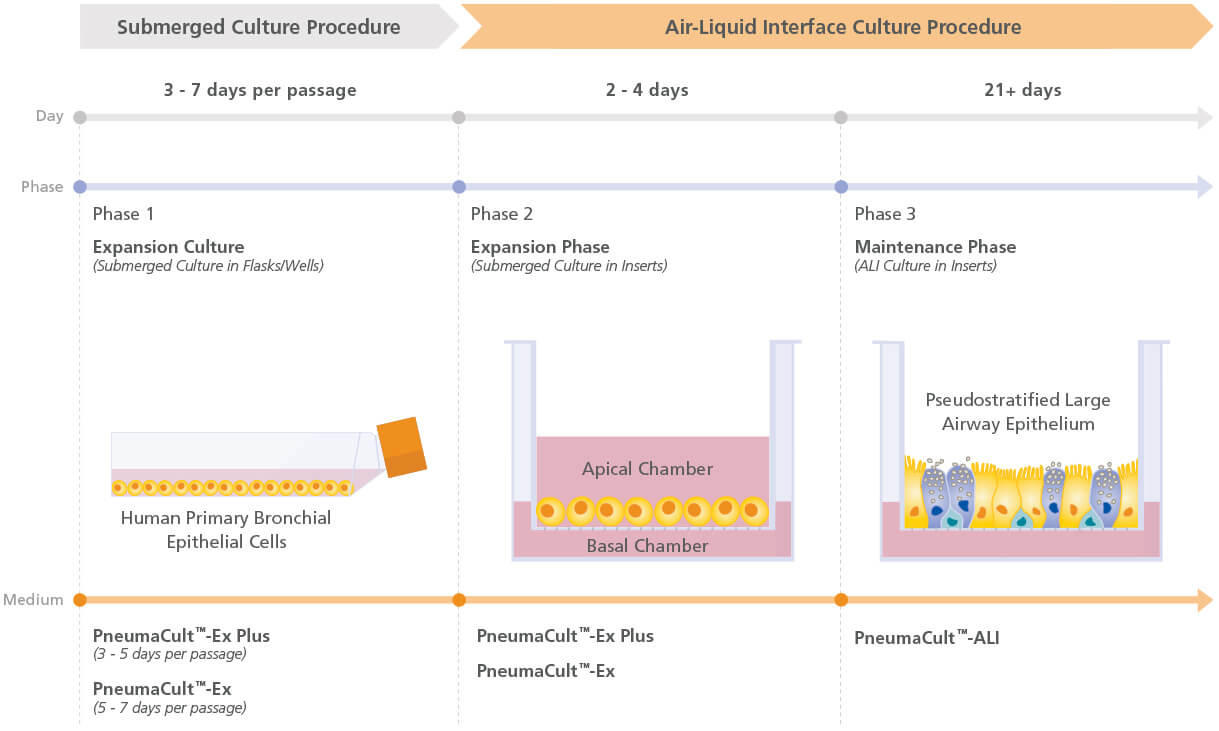
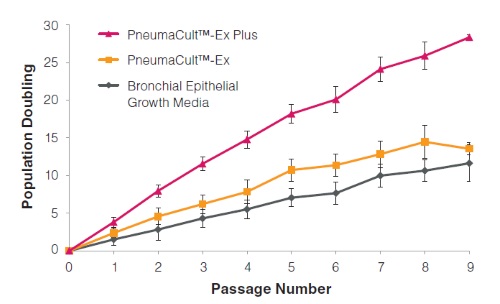
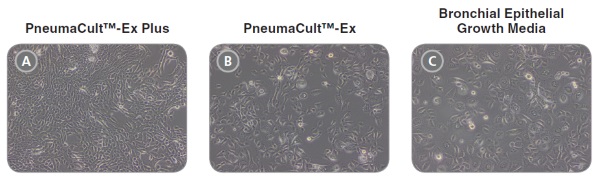
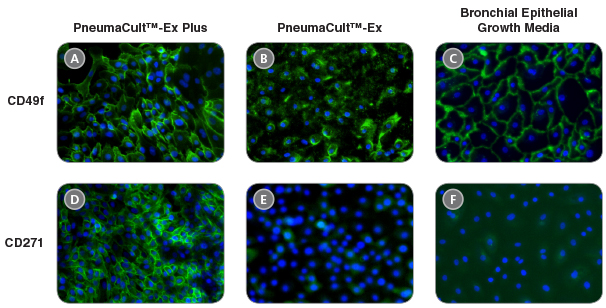
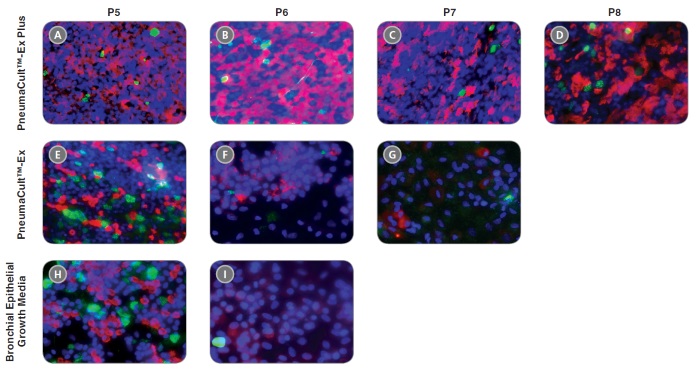
PneumaCult™-Ex Plus Medium is a defined, serum- and BPE-free cell culture medium that supports more expansion of primary human airway epithelial cells at each passage, compared to other commercially available expansion media. This medium also supports at least two additional passages of cell expansion with better differentiation potential, defined as the ability to form a pseudostratified mucociliary epithelium at the air-liquid interface (ALI) using PneumaCult™-ALI Medium (Catalog #05001) or a cuboidal epithelium using PneumaCult™-ALI-S Medium (Catalog #05050).
PneumaCult™-Ex Plus and either PneumaCult™-ALI or PneumaCult™-ALI-S constitute a fully integrated BPE-free culture system for in vitro human airway modeling. This robust and defined system is a valuable tool for basic respiratory research, toxicity studies, and drug development.
• A defined, serum- and BPE-free cell culture medium that delivers consistent performance
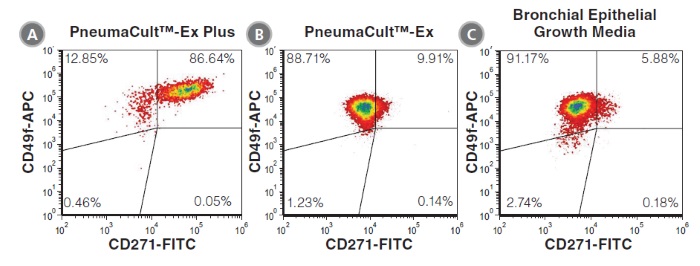
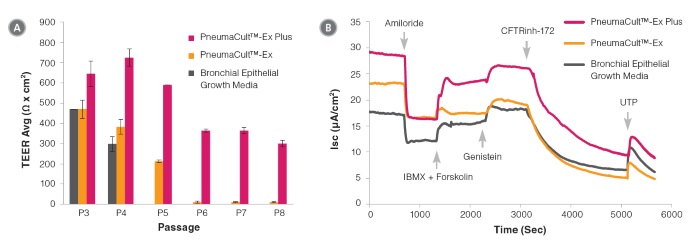
• PneumaCult™-Ex Plus Medium supports more cell expansion at each passage compared to other commercially available expansion media
• When used together with PneumaCult™-ALI Medium or PneumaCult™-ALI-S Medium, PneumaCult™-Ex Plus Medium supports better ALI differentiation potential even after extended passaging compared to other commercially available expansion media
- PneumaCult™-Ex Plus Basal Medium, 490 mL
- PneumaCult™-Ex Plus 50X Supplement, 10 mL
Cell Culture, Expansion, Maintenance
数据及文献
Publications (13)
Physiological reports 2020 oct
Effect of apical chloride concentration on the measurement of responses to CFTR modulation in airway epithelia cultured from nasal brushings.
P. E. Bratcher et al.
Abstract
INTRODUCTION One method for assessing the in vitro response to CFTR-modulating compounds is by analysis of epithelial monolayers in an Ussing chamber, where the apical and basolateral surfaces are isolated and the potential difference, short-circuit current, and transepithelial resistance can be monitored. The effect of a chloride ion gradient across airway epithelia on transepithelial chloride transport and the magnitude of CFTR modulator efficacy were examined. METHODS CFTR-mediated changes in the potential difference and transepithelial currents of primary human nasal epithelial cell cultures were quantified in Ussing chambers with either symmetrical solutions or reduced chloride solutions in the apical chamber. CFTR activity in homozygous F508del CFTR epithelia was rescued by treatment with VX-661, C4/C18, 4-phenylbutyrate (4-PBA) for 24 hr at 37°C or by incubation at 29°C for 48 hr. RESULTS Imposing a chloride gradient increased CFTR-mediated and CaCC-mediated ion transport. Treatment of F508del CFTR homozygous cells with CFTR modulating compounds increased CFTR activity, which was significantly more evident in the presence of a chloride gradient. This observation was recapitulated with temperature-mediated F508del CFTR correction. CONCLUSIONS Imposing a chloride gradient during Ussing chamber measurements resulted in increased CFTR-mediated ion transport in expanded non-CF and F508del CFTR homozygous epithelia. In F508del CFTR homozygous epithelia, the magnitude of response to CFTR modulating compounds or low temperature was greater when assayed with a chloride gradient compared to symmetrical chloride, resulting in an apparent increase in measured efficacy. Future work may direct which methodologies utilized to quantify CFTR modulator response in vitro are most appropriate for the estimation of in vivo efficacy.
Journal of clinical medicine 2020 nov
A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia.
J. L. Coles et al.
Abstract
Air-liquid interface (ALI) culture of nasal epithelial cells is a valuable tool in the diagnosis and research of primary ciliary dyskinesia (PCD). Ex vivo samples often display secondary dyskinesia from cell damage during sampling, infection or inflammation confounding PCD diagnostic results. ALI culture enables regeneration of healthy cilia facilitating differentiation of primary from secondary ciliary dyskinesia. We describe a revised ALI culture method adopted from April 2018 across three collaborating PCD diagnostic sites, including current University Hospital Southampton COVID-19 risk mitigation measures, and present results. Two hundred and forty nasal epithelial cell samples were seeded for ALI culture and 199 (82.9{\%}) were ciliated. Fifty-four of 83 (63.9{\%}) ex vivo samples which were originally equivocal or insufficient provided diagnostic information following in vitro culture. Surplus basal epithelial cells from 181 nasal brushing samples were frozen in liquid nitrogen; 39 samples were ALI-cultured after cryostorage and all ciliated. The ciliary beat patterns of ex vivo samples (by high-speed video microscopy) were recapitulated, scanning electron microscopy demonstrated excellent ciliation, and cilia could be immuno-fluorescently labelled (anti-alpha-tubulin and anti-RSPH4a) in representative cases that were ALI-cultured after cryostorage. In summary, our ALI culture protocol provides high ciliation rates across three centres, minimising patient recall for repeat brushing biopsies and improving diagnostic certainty. Cryostorage of surplus diagnostic samples was successful, facilitating PCD research.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2020 jun
Recapitulating idiopathic pulmonary fibrosis related alveolar epithelial dysfunction in a human iPSC-derived air-liquid interface model.
E. Schruf et al.
Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal disease of unknown cause that is characterized by progressive fibrotic lung remodeling. An abnormal emergence of airway epithelial-like cells within the alveolar compartments of the lung, herein termed bronchiolization, is often observed in IPF. However, the origin of this dysfunctional distal lung epithelium remains unknown due to a lack of suitable human model systems. In this study, we established a human induced pluripotent stem cell (iPSC)-derived air-liquid interface (ALI) model of alveolar epithelial type II (ATII)-like cell differentiation that allows us to investigate alveolar epithelial progenitor cell differentiation in vitro. We treated this system with an IPF-relevant cocktail (IPF-RC) to mimic the pro-fibrotic cytokine milieu present in IPF lungs. Stimulation with IPF-RC during differentiation increases secretion of IPF biomarkers and RNA sequencing (RNA-seq) of these cultures reveals significant overlap with human IPF patient data. IPF-RC treatment further impairs ATII differentiation by driving a shift toward an airway epithelial-like expression signature, providing evidence that a pro-fibrotic cytokine environment can influence the proximo-distal differentiation pattern of human lung epithelial cells. In conclusion, we show for the first time, the establishment of a human model system that recapitulates aspects of IPF-associated bronchiolization of the lung epithelium in vitro.
American journal of physiology. Lung cellular and molecular physiology 2020 jun
Real-time measurement of cellular bioenergetics in fully differentiated human nasal epithelial cells grown at air-liquid-interface.
E. Mavin et al.
Abstract
Shifts in cellular metabolic phenotypes have the potential to cause disease-driving processes in respiratory disease. The respiratory epithelium is particularly susceptible to metabolic shifts in disease, but our understanding of these processes is limited by the incompatibility of the technology required to measure metabolism in real-time with the cell culture platforms used to generate differentiated respiratory epithelial cell types. Thus, to date, our understanding of respiratory epithelial metabolism has been restricted to that of basal epithelial cells in submerged culture, or via indirect end point metabolomics readouts in lung tissue. Here we present a novel methodology using the widely available Seahorse Analyzer platform to monitor real-time changes in the cellular metabolism of fully differentiated primary human airway epithelial cells grown at air-liquid interface (ALI). We show increased glycolytic, but not mitochondrial, ATP production rates in response to physiologically relevant increases in glucose availability. We also show that pharmacological inhibition of lactate dehydrogenase is able to reduce glucose-induced shifts toward aerobic glycolysis. This method is timely given the recent advances in our understanding of new respiratory epithelial subtypes that can only be observed in vitro through culture at ALI and will open new avenues to measure real-time metabolic changes in healthy and diseased respiratory epithelium, and in turn the potential for the development of novel therapeutics targeting metabolic-driven disease phenotypes.
Nature communications 2020 dec
Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium.
S. P. Sajuthi et al.
Abstract
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2, an emerging virus that utilizes host proteins ACE2 and TMPRSS2 as entry factors. Understanding the factors affecting the pattern and levels of expression of these genes is important for deeper understanding of SARS-CoV-2 tropism and pathogenesis. Here we explore the role of genetics and co-expression networks in regulating these genes in the airway, through the analysis of nasal airway transcriptome data from 695 children. We identify expression quantitative trait loci for both ACE2 and TMPRSS2, that vary in frequency across world populations. We find TMPRSS2 is part of a mucus secretory network, highly upregulated by type 2 (T2) inflammation through the action of interleukin-13, and that the interferon response to respiratory viruses highly upregulates ACE2 expression. IL-13 and virus infection mediated effects on ACE2 expression were also observed at the protein level in the airway epithelium. Finally, we define airway responses to common coronavirus infections in children, finding that these infections generate host responses similar to other viral species, including upregulation of IL6 and ACE2. Our results reveal possible mechanisms influencing SARS-CoV-2 infectivity and COVID-19 clinical outcomes.
BMC pulmonary medicine 2020 aug
Effect of sub-chronic exposure to cigarette smoke, electronic cigarette and waterpipe on human lung epithelial barrier function.
B. Ghosh et al.
Abstract
BACKGROUND Taking into consideration a recent surge of a lung injury condition associated with electronic cigarette use, we devised an in vitro model of sub-chronic exposure of human bronchial epithelial cells (HBECs) in air-liquid interface, to determine deterioration of epithelial cell barrier from sub-chronic exposure to cigarette smoke (CS), e-cigarette aerosol (EC), and tobacco waterpipe exposures (TW). METHODS Products analyzed include commercially available e-liquid, with 0{\%} or 1.2{\%} concentration of nicotine, tobacco blend (shisha), and reference-grade cigarette (3R4F). In one set of experiments, HBECs were exposed to EC (0 and 1.2{\%}), CS or control air for 10 days using 1 cigarette/day. In the second set of experiments, exposure of pseudostratified primary epithelial tissue to TW or control air exposure was performed 1-h/day, every other day, until 3 exposures were performed. After 16-18 h of last exposure, we investigated barrier function/structural integrity of the epithelial monolayer with fluorescein isothiocyanate-dextran flux assay (FITC-Dextran), measurements of trans-electrical epithelial resistance (TEER), assessment of the percentage of moving cilia, cilia beat frequency (CBF), cell motion, and quantification of E-cadherin gene expression by reverse-transcription quantitative polymerase chain reaction (RT-qPCR). RESULTS When compared to air control, CS increased fluorescence (FITC-Dextran assay) by 5.6 times, whereby CS and EC (1.2{\%}) reduced TEER to 49 and 60{\%} respectively. CS and EC (1.2{\%}) exposure reduced CBF to 62 and 59{\%}, and cilia moving to 47 and 52{\%}, respectively, when compared to control air. CS and EC (1.2{\%}) increased cell velocity compared to air control by 2.5 and 2.6 times, respectively. The expression of E-cadherin reduced to 39{\%} of control air levels by CS exposure shows an insight into a plausible molecular mechanism. Altogether, EC (0{\%}) and TW exposures resulted in more moderate decreases in epithelial integrity, while EC (1.2{\%}) substantially decreased airway epithelial barrier function comparable with CS exposure. CONCLUSIONS The results support a toxic effect of sub-chronic exposure to EC (1.2{\%}) as evident by disruption of the bronchial epithelial cell barrier integrity, whereas further research is needed to address the molecular mechanism of this observation as well as TW and EC (0{\%}) toxicity in chronic exposures.
View All Publications