NeuroCult™ SM1 Neuronal Supplement
Supplement (50X) for the serum-free culture of neurons
概要
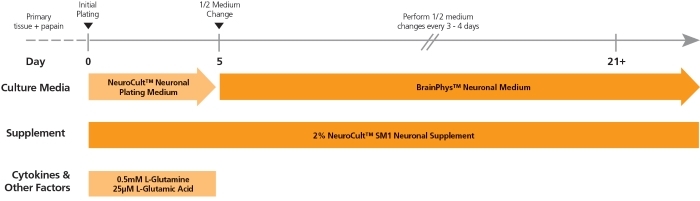
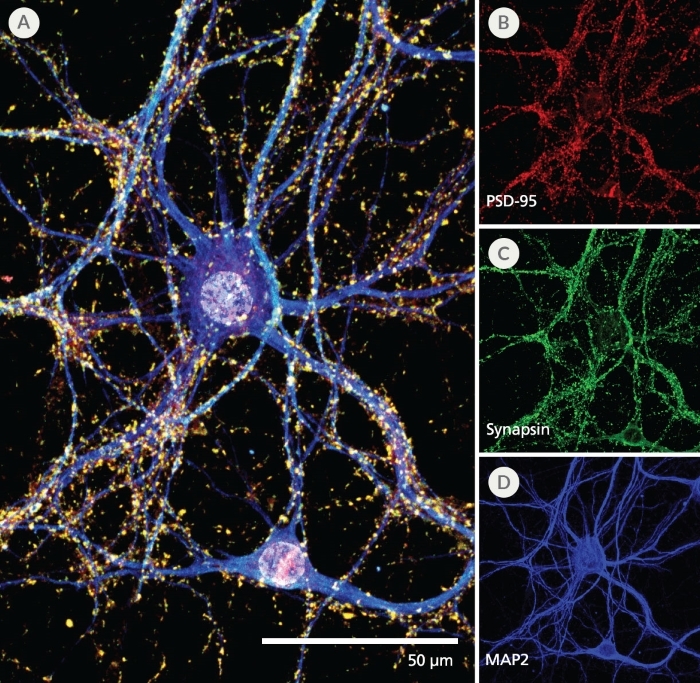
NeuroCult™ SM1 (STEMCELL Modified-1) Neuronal Supplement is a serum-free supplement for the culture of primary or pluripotent stem cell (PSC)-derived neurons. NeuroCult™ SM1 is used in combination with a basal neural medium such as BrainPhys™ Neuronal Medium (Catalog #05790) or as a serum-replacement supplement for various customizable applications wherever B27 is used.
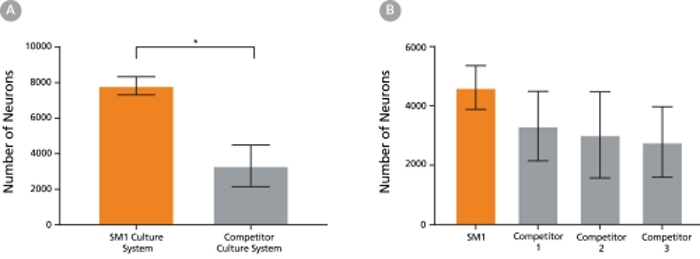
The NeuroCult™ SM1 formulation was developed based on the published formulation of Brewer’s B27 supplement. It has been optimized to reproducibly support increased survival and maturation of functional neurons in both short- and long-term cultures with minimal glial cell contamination. NeuroCult™ SM1 is also a component of several BrainPhys™ kits for primary and PSC-derived neuronal cell culture. For further details and protocols, refer to the Product Information Sheet (PIS) for BrainPhys™, available at www.stemcell.com or contact us to request a copy.
• Versatile cell culture supplement
• Optimized, serum-free formulation
• Raw materials rigorously screened to minimize lot-to-lot consistency
• Antioxidants
• Vitamin A
• Insulin
• Other ingredients
Neural Cells, PSC-Derived, Neurons, Pluripotent Stem Cells
Cell Culture, Differentiation, Maintenance
Neuroscience, Stem Cell Biology
数据及文献
Publications (50)
Viruses 2020 mar
Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures.
V. Sundaramoorthy et al.
Abstract
Rabies is a zoonotic neurological infection caused by lyssavirus that continues to result in devastating loss of human life. Many aspects of rabies pathogenesis in human neurons are not well understood. Lack of appropriate ex-vivo models for studying rabies infection in human neurons has contributed to this knowledge gap. In this study, we utilize advances in stem cell technology to characterize rabies infection in human stem cell-derived neurons. We show key cellular features of rabies infection in our human neural cultures, including upregulation of inflammatory chemokines, lack of neuronal apoptosis, and axonal transmission of viruses in neuronal networks. In addition, we highlight specific differences in cellular pathogenesis between laboratory-adapted and field strain lyssavirus. This study therefore defines the first stem cell-derived ex-vivo model system to study rabies pathogenesis in human neurons. This new model system demonstrates the potential for enabling an increased understanding of molecular mechanisms in human rabies, which could lead to improved control methods.
Frontiers in bioengineering and biotechnology 2020
Maturation of Human Pluripotent Stem Cell-Derived Cerebellar Neurons in the Absence of Co-culture.
T. P. Silva et al.
Abstract
The cerebellum plays a critical role in all vertebrates, and many neurological disorders are associated with cerebellum dysfunction. A major limitation in cerebellar research has been the lack of adequate disease models. As an alternative to animal models, cerebellar neurons differentiated from pluripotent stem cells have been used. However, previous studies only produced limited amounts of Purkinje cells. Moreover, in vitro generation of Purkinje cells required co-culture systems, which may introduce unknown components to the system. Here we describe a novel differentiation strategy that uses defined medium to generate Purkinje cells, granule cells, interneurons, and deep cerebellar nuclei projection neurons, that self-formed and differentiated into electrically active cells. Using a defined basal medium optimized for neuronal cell culture, we successfully promoted the differentiation of cerebellar precursors without the need for co-culturing. We anticipate that our findings may help developing better models for the study of cerebellar dysfunctions, while providing an advance toward the development of autologous replacement strategies for treating cerebellar degenerative diseases.
American journal of human genetics 2019
Mutations in ACTL6B Cause Neurodevelopmental Deficits and Epilepsy and Lead to Loss of Dendrites in Human Neurons.
S. Bell et al.
Abstract
We identified individuals with variations in ACTL6B, a component of the chromatin remodeling machinery including the BAF complex. Ten individuals harbored bi-allelic mutations and presented with global developmental delay, epileptic encephalopathy, and spasticity, and ten individuals with de novo heterozygous mutations displayed intellectual disability, ambulation deficits, severe language impairment, hypotonia, Rett-like stereotypies, and minor facial dysmorphisms (wide mouth, diastema, bulbous nose). Nine of these ten unrelated individuals had the identical de novo c.1027G{\textgreater}A (p.Gly343Arg) mutation. Human-derived neurons were generated that recaptured ACTL6B expression patterns in development from progenitor cell to post-mitotic neuron, validating the use of this model. Engineered knock-out of ACTL6B in wild-type human neurons resulted in profound deficits in dendrite development, a result recapitulated in two individuals with different bi-allelic mutations, and reversed on clonal genetic repair or exogenous expression of ACTL6B. Whole-transcriptome analyses and whole-genomic profiling of the BAF complex in wild-type and bi-allelic mutant ACTL6B neural progenitor cells and neurons revealed increased genomic binding of the BAF complex in ACTL6B mutants, with corresponding transcriptional changes in several genes including TPPP and FSCN1, suggesting that altered regulation of some cytoskeletal genes contribute to altered dendrite development. Assessment of bi-alleic and heterozygous ACTL6B mutations on an ACTL6B knock-out human background demonstrated that bi-allelic mutations mimic engineered deletion deficits while heterozygous mutations do not, suggesting that the former are loss of function and the latter are gain of function. These results reveal a role for ACTL6B in neurodevelopment and implicate another component of chromatin remodeling machinery in brain disease.
Scientific reports 2018 MAY
Acute Physiology and Neurologic Outcomes after Brain Injury in SCOP/PHLPP1 KO Mice.
T. C. Jackson et al.
Abstract
Suprachiasmatic nucleus circadian oscillatory protein (SCOP) (a.k.a. PHLPP1) regulates long-term memory consolidation in the brain. Using a mouse model of controlled cortical impact (CCI) we tested if (1) brain tissue levels of SCOP/PHLPP1 increase after a traumatic brain injury (TBI), and (2) if SCOP/PHLPP1 gene knockout (KO) mice have improved (or worse) neurologic outcomes. Blood chemistry (pH, pCO2, pO2, pSO2, base excess, sodium bicarbonate, and osmolarity) and arterial pressure (MAP) differed in isoflurane anesthetized WT vs. KOs at baseline and up to 1 h post-injury. CCI injury increased cortical/hippocampal SCOP/PHLPP1 levels in WTs 7d and 14d post-injury. Injured KOs had higher brain tissue levels of phosphorylated AKT (pAKT) in cortex (14d post-injury), and higher levels of phosphorylated MEK (pMEK) in hippocampus (7d and 14d post-injury) and in cortex (7d post-injury). Consistent with an important role of SCOP/PHLPP1 on memory function, injured-KOs had near normal performance on the probe trial of the Morris water maze, whereas injured-WTs were impaired. CA1/CA3 hippocampal survival was lower in KOs vs. WTs 24 h post-injury but equivalent by 7d. No difference in 21d cortical lesion volume was detected. SCOP/PHLPP1 overexpression in cultured rat cortical neurons had no effect on 24 h cell death after a mechanical stretch-injury.
Cell reports 2018 JUN
CaMKII Metaplasticity Drives Abeta$ Oligomer-Mediated Synaptotoxicity.
P. Opazo et al.
Abstract
Alzheimer's disease (AD) is emerging as a synaptopathology driven by metaplasticity. Indeed, reminiscent of metaplasticity, oligomeric forms of the amyloid-beta$ peptide (oAbeta$) prevent induction of long-term potentiation (LTP) via the prior activation of GluN2B-containing NMDA receptors (NMDARs). However, the downstream Ca2+-dependent signaling molecules that mediate aberrant metaplasticity are unknown. In this study, we show that oAbeta$ promotes the activation of Ca2+/calmodulin-dependent kinase II (CaMKII) via GluN2B-containing NMDARs. Importantly, we find that CaMKII inhibition rescues both the LTP impairment and the dendritic spine loss mediated by oAbeta$. Mechanistically resembling metaplasticity, oAbeta$ prevents subsequent rounds of plasticity from inducing CaMKII T286 autophosphorylation, as well as the associated anchoring and accumulation of synaptic AMPA receptors (AMPARs). Finally, prolonged oAbeta$ treatment-induced CaMKII misactivation leads to dendritic spine loss via the destabilization of surface AMPARs. Thus, our study demonstrates that oAbeta$ engages synaptic metaplasticity via aberrant CaMKII activation.
Scientific reports 2018 JUL
Toxicological evaluation of convulsant and anticonvulsant drugs in human induced pluripotent stem cell-derived cortical neuronal networks using an MEA system.
A. Odawara et al.
Abstract
Functional evaluation assays using human induced pluripotent stem cell (hiPSC)-derived neurons can predict the convulsion toxicity of new drugs and the neurological effects of antiepileptic drugs. However, differences in responsiveness depending on convulsant type and antiepileptic drugs, and an evaluation index capable of comparing in vitro responses with in vivo responses are not well known. We observed the difference in synchronized burst patterns in the epileptiform activities induced by pentylentetrazole (PTZ) and 4-aminopryridine (4-AP) with different action mechanisms using multi-electrode arrays (MEAs); we also observed that 100 µM of the antiepileptic drug phenytoin suppressed epileptiform activities induced by PTZ, but increased those induced by 4-AP. To compare in vitro results with in vivo convulsive responses, frequency analysis of below 250 Hz, excluding the spike component, was performed. The in vivo convulsive firing enhancement of the high gamma$ wave and beta$ wave component were observed remarkably in in vitro hiPSC-derived neurons with astrocytes in co-culture. MEA measurement of hiPSC-derived neurons in co-culture with astrocytes and our analysis methods, including frequency analysis, appear effective for predicting convulsion toxicity, side effects, and their mechanism of action as well as the comparison of convulsions induced in vivo.
View All Publications