Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R
This antibody clone has been verified for labeling human ES and iPS cells grown in TeSR™-E8™ (Catalog #05940), mTeSR™1 (Catalog #85850), and TeSR™2 (Catalog #05860), and for purity assessments of cells isolated with EasySep™ kits, including EasySep™ Human ES/iPS Cell TRA-1-60 Positive Selection Kit (Catalog #18166; partial blocking may be observed) and EasySep™ hESC/hiPSC SSEA-4 Positive Selection Kit (Catalog #18165).
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R | 60064 | All | English |
| Product Information Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488 | 60064AD, 60064AD.1 | All | English |
| Product Information Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Biotin | 60064BT | All | English |
| Product Information Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE | 60064PE, 60064PE.1 | All | English |
| Safety Data Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R | 60064 | All | English |
| Safety Data Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488 | 60064AD, 60064AD.1 | All | English |
| Safety Data Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Biotin | 60064BT | All | English |
| Safety Data Sheet | Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE | 60064PE, 60064PE.1 | All | English |
Data
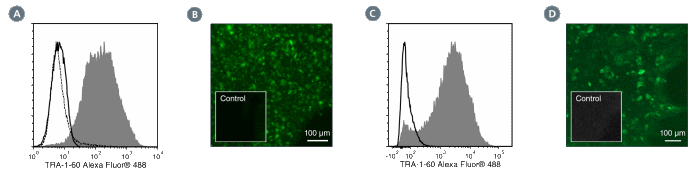
Figure 1. Data for Alexa Fluor® 488-Conjugated
(A) Flow cytometry analysis of human ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control, dashed line histogram) labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488. Labeling of human ES cells with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, Alexa Fluor® 488 is shown (Catalog #60069AD, solid line histogram).
(B) Human ES cells were cultured in mTeSR™1 on BD Matrigel™-coated glass slides, then fixed and stained with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488. Inset shows cells labeled with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, Alexa Fluor® 488.
(C) Flow cytometry analysis of human iPS cells labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488 (filled histogram) or Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, Alexa Fluor® 488 (open histogram).
(D) Human iPS cells were cultured in mTeSR™1 on BD Matrigel™-coated glass slides, then fixed and stained with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Alexa Fluor® 488. Inset shows cells labeled with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, Alexa Fluor® 488.
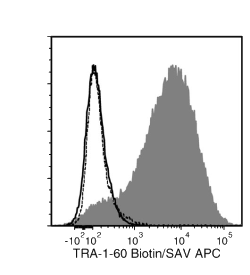
Figure 2. Data for Biotin-Conjugated
Flow cytometry analysis of human ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control, dashed line histogram) labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, Biotin followed by streptavidin (SAV) APC (filled histogram). Labeling human ES cells with a mouse IgM, kappa biotin isotype control antibody followed by SAV APC is shown (solid line histogram).
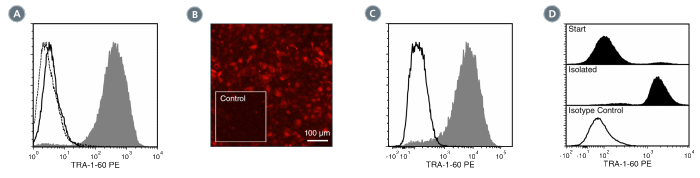
Figure 3. Data for PE-Conjugated
(A) Flow cytometry analysis of human embryonic stem (ES) cells (filled histogram) or HT1080 fibrosarcoma cells (negative control, dashed line histogram) labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE. Labeling of human ES cells with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, PE is shown (Catalog #60069PE, solid line histogram).
(B) Human ES cells were cultured in mTeSR™1 on BD Matrigel™-coated glass slides, then fixed and stained with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE. Inset shows cells labeled with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, PE.
(C) Flow cytometry analysis of human iPS cells labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE (filled histogram) or Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, PE (open histogram).
(D) Flow cytometry analysis of human ES cells isolated with the EasySep™ Human ES/iPS Cell TRA-1-60 Positive Selection Kit from a mixed population of ES cells and HT1080 fibrosarcoma cells and labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, PE. Histograms show labeling of the starting population containing ~5% ES cells (Start) and the isolated cells (Isolated). Labeling with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, PE is shown in the bottom panel (open histogram).
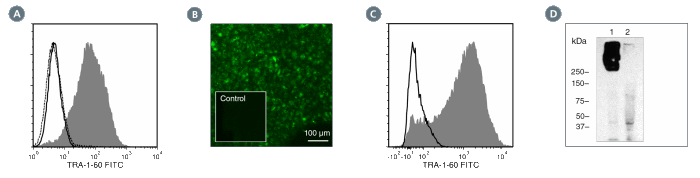
Figure 4. Data for Unconjugated
(A) Flow cytometry analysis of human ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control, dashed line histogram) labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, followed by goat anti-mouse IgG, FITC. Labeling of human ES cells with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30 (Catalog #60069) followed by goat anti-mouse IgG, FITC is shown (solid line histogram).
(B) Human ES cells were cultured in mTeSR™1 on BD Matrigel™-coated glass slides, then fixed and stained with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, followed by goat anti-mouse IgG, FITC. Inset shows cells labeled with Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, followed by goat anti-mouse IgG, FITC.
(C) Flow cytometry analysis of human iPS cells labeled with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R, followed by goat anti-mouse IgG, FITC (filled histogram) or Mouse IgM, kappa Isotype Control Antibody, Clone MM-30, followed by goat anti-mouse IgG, FITC (open histogram).
(D) Western blot analysis of denatured/reduced cell lysates from human ES cells (lane 1) or HT1080 fibrosarcoma cells (negative control, lane 2) with Anti-Human TRA-1-60 Antibody, Clone TRA-1-60R.