Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20 | 60093, 60093.1 | All | English |
| Product Information Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, Alexa Fluor® 488 | 60093AD, 60093AD.1 | All | English |
| Product Information Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, PE | 60093PE, 60093PE.1 | All | English |
| Safety Data Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20 | 60093, 60093.1 | All | English |
| Safety Data Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, Alexa Fluor® 488 | 60093AD, 60093AD.1 | All | English |
| Safety Data Sheet | Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, PE | 60093PE, 60093PE.1 | All | English |
Data
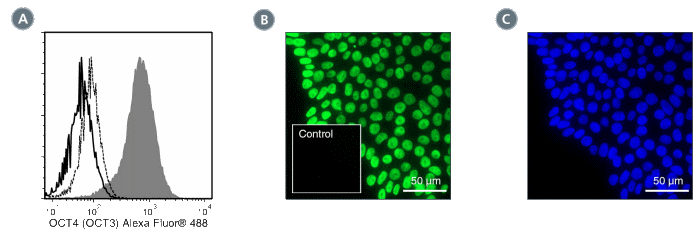
Figure 1. Data for Alexa Fluor® 488-Conjugated
(A) Flow cytometry analysis of ES cells cultured with mTeSR™1 on Corning® Matrigel®. The ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control; dashed line histogram) were fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, Alexa Fluor® 488. Labeling of the ES cells with Mouse IgG2b, kappa Isotype Control Antibody, Clone MPC-11, Alexa Fluor® 488 (Catalog #60072AD) is shown (solid line histogram). (B) Human ES cells were cultured with TeSR™-E8™ on glass coverslips coated with Vitronectin XF™ (Catalog #07180), then fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, Alexa Fluor® 488. Inset shows labeling of human ES cells with Mouse IgG2b, kappa Isotype Control Antibody, Clone MPC-11, Alexa Fluor® 488. (C) DAPI counterstaining of the cells shown in figure (B); nuclear localization of the OCT4 (OCT3) marker is evident.
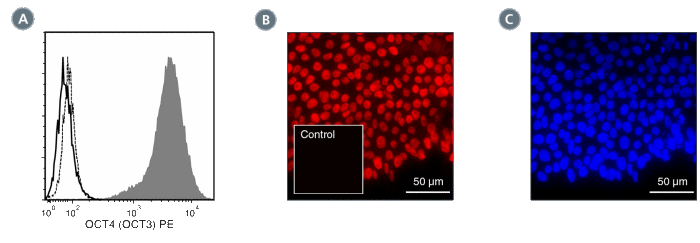
Figure 2. Data for PE-Conjugated
(A) Flow cytometry analysis of human ES cells cultured with mTeSR™1 on Corning® Matrigel®. The ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control; dashed line histogram) were fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, PE. Labeling of the ES cells with Mouse IgG2b, kappa Isotype Control Antibody, Clone MPC-11, PE (Catalog #60072PE) is shown (solid line histogram). (B) Human ES cells were cultured with TeSR™-E8™ on glass coverslips coated with Vitronectin XF™ (Catalog #07180), then fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, PE. Inset shows labeling of human ES cells with Mouse IgG2b, kappa Isotype Control Antibody, Clone MPC-11, PE. (C) DAPI counterstaining of the cells shown in figure (B); nuclear localization of the OCT4 (OCT3) marker is evident.
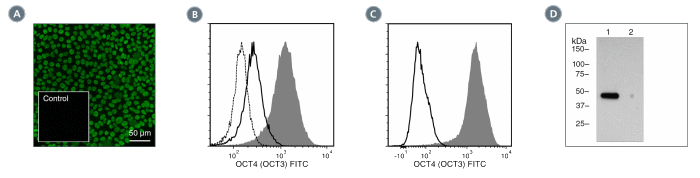
Figure 3. Data for Unconjugated
(A) Human induced pluripotent stem (iPS) cells were cultured with TeSR™-E8™ on glass coverslips coated with Vitronectin XF™ (Catalog #07180), then fixed and stained with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, followed by goat anti-mouse IgG, FITC. Inset shows cells labeled with a mouse IgG2b, kappa isotype control antibody followed by goat anti-mouse IgG, FITC.
(B) Flow cytometry analysis of human embryonic stem (ES) cells cultured with mTeSR™1 on Corning® Matrigel®. The ES cells (filled histogram) or HT1080 fibrosarcoma cells (negative control, dashed line histogram) were fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, followed by goat anti-mouse IgG, FITC. Labeling of the ES cells with a mouse IgG2b, kappa isotype control antibody followed by goat anti-mouse IgG, FITC is shown (solid line histogram). (C) Flow cytometry analysis of human iPS cells cultured with TeSR™-E8™ on Vitronectin XF™. The cells were fixed and labeled with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20, followed by goat anti-mouse IgG, FITC (filled histogram) or a mouse IgG2b, kappa isotype control antibody followed by goat anti-mouse IgG, FITC (solid line histogram). (D) Western blot analysis of denatured/reduced cell lysates with Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20. Lane 1, human ES cells cultured with mTeSR™1 on Corning® Matrigel®, lane 2 (negative control), mouse E13.5 neural progenitor cells cultured with NeuroCult™ Proliferation Kit (Mouse, Catalog #05702).