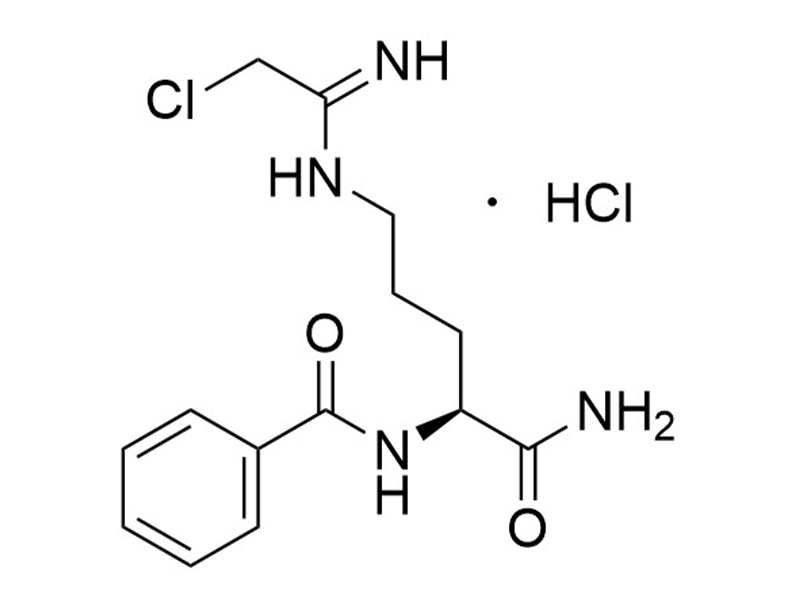
Cl-Amidine
Inhibits protein-arginine deiminase (PAD)
概要
Cl-Amidine is a protein-arginine deiminase (PAD) inhibitor that irreversibly inactivates four subtypes of PAD (kinact/Ki = 37,000/PAD1, 1,200/PAD2, 2,000/PAD3, and 13,000/PAD4 M-1min-1; Knuckley et al.; Luo et al.; Slack et al.) by modifying the enzyme’s active site. This product is supplied as the hydrochloride salt of the molecule.
IMMUNOLOGY
· Inhibits neutrophil extracellular traps formation in neutrophils (Knight et al.).
· Prevents hypercitrullination of histone H3 in neutrophils (Knight et al.).
CANCER RESEARCH
· Exhibits cytotoxic effects toward human leukemia (IC50 = 0.25 μM), breast (IC50 = 0.05 μM), and colon (IC50 = 1 μM) cancer cell lines (Slack et al.).
IMMUNOLOGY
· Inhibits neutrophil extracellular traps formation in neutrophils (Knight et al.).
· Prevents hypercitrullination of histone H3 in neutrophils (Knight et al.).
CANCER RESEARCH
· Exhibits cytotoxic effects toward human leukemia (IC50 = 0.25 μM), breast (IC50 = 0.05 μM), and colon (IC50 = 1 μM) cancer cell lines (Slack et al.).
Cell Type
Cancer Cells and Cell Lines, Granulocytes and Subsets
Area of Interest
Cancer Research, Immunology
CAS Number
1373232-26-8
Chemical Formula
C14H19ClN4O2 • HCl
Molecular Weight
347.2 g/mol
Purity
≥ 95%
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Cl-Amidine (Hydrochloride) | 100-0518, 100-0519 | All | English |
| Safety Data Sheet | Cl-Amidine (Hydrochloride) | 100-0518, 100-0519 | All | English |
数据及文献
Publications (5)
Annals of the rheumatic diseases 2015 dec
Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice.
Abstract
Abstract
OBJECTIVES An imbalance between neutrophil extracellular trap (NET) formation and degradation has been described in systemic lupus erythematosus (SLE), potentially contributing to autoantigen externalisation, type I interferon synthesis and endothelial damage. We have demonstrated that peptidylarginine deiminase (PAD) inhibition reduces NET formation and protects against lupus-related vascular damage in the New Zealand Mixed model of lupus. However, another strategy for inhibiting NETs--knockout of NOX2--accelerates lupus in a different murine model, MRL/lpr. Here, we test the effects of PAD inhibition on MRL/lpr mice in order to clarify whether some NET inhibitory pathways may be consistently therapeutic across models of SLE. METHODS NET formation and autoantibodies to NETs were characterised in lupus-prone MRL/lpr mice. MRL/lpr mice were also treated with two different PAD inhibitors, Cl-amidine and the newly described BB-Cl-amidine. NET formation, endothelial function, interferon signature, nephritis and skin disease were examined in treated mice. RESULTS Neutrophils from MRL/lpr mice demonstrate accelerated NET formation compared with controls. MRL/lpr mice also form autoantibodies to NETs and have evidence of endothelial dysfunction. PAD inhibition markedly improves endothelial function, while downregulating the expression of type I interferon-regulated genes. PAD inhibition also reduces proteinuria and immune complex deposition in the kidneys, while protecting against skin disease. CONCLUSIONS PAD inhibition reduces NET formation, while protecting against lupus-related damage to the vasculature, kidneys and skin in various lupus models. The strategy by which NETs are inhibited will have to be carefully considered if human studies are to be undertaken.
The Journal of clinical investigation 2013 jul
Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus.
Abstract
Abstract
Recent evidence suggests that enhanced neutrophil extracellular trap (NET) formation activates plasmacytoid dendritic cells and serves as a source of autoantigens in SLE. We propose that aberrant NET formation is also linked to organ damage and to the premature vascular disease characteristic of human SLE. Here, we demonstrate enhanced NET formation in the New Zealand mixed 2328 (NZM) model of murine lupus. NZM mice also developed autoantibodies to NETs as well as the ortholog of human cathelicidin/LL37 (CRAMP), a molecule externalized in the NETs. NZM mice were treated with Cl-amidine, an inhibitor of peptidylarginine deiminases (PAD), to block NET formation and were evaluated for lupus-like disease activity, endothelial function, and prothrombotic phenotype. Cl-amidine treatment inhibited NZM NET formation in vivo and significantly altered circulating autoantibody profiles and complement levels while reducing glomerular IgG deposition. Further, Cl-amidine increased the differentiation capacity of bone marrow endothelial progenitor cells, improved endothelium-dependent vasorelaxation, and markedly delayed time to arterial thrombosis induced by photochemical injury. Overall, these findings suggest that PAD inhibition can modulate phenotypes crucial for lupus pathogenesis and disease activity and may represent an important strategy for mitigating cardiovascular risk in lupus patients.
Cellular and molecular life sciences : CMLS 2011 feb
Protein arginine deiminase 4: a target for an epigenetic cancer therapy.
Abstract
Abstract
The recent approvals of anticancer therapeutic agents targeting the histone deacetylases and DNA methyltransferases have highlighted the important role that epigenetics plays in human diseases, and suggested that the factors controlling gene expression are novel drug targets. Protein arginine deiminase 4 (PAD4) is one such target because its effects on gene expression parallel those observed for the histone deacetylases. We demonstrated that F- and Cl-amidine, two potent PAD4 inhibitors, display micromolar cytotoxic effects towards several cancerous cell lines (HL-60, MCF7 and HT-29); no effect was observed in noncancerous lines (NIH 3T3 and HL-60 granulocytes). These compounds also induced the differentiation of HL-60 and HT29 cells. Finally, these compounds synergistically potentiated the cell killing effects of doxorubicin. Taken together, these findings suggest PAD4 inhibition as a novel epigenetic approach for the treatment of cancer, and suggest that F- and Cl-amidine are candidate therapeutic agents for this disease.
Biochemistry 2010 jun
Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3.
Abstract
Abstract
Protein citrullination has been shown to regulate numerous physiological pathways (e.g., the innate immune response and gene transcription) and is, when dysregulated, known to be associated with numerous human diseases, including cancer, rheumatoid arthritis, and multiple sclerosis. This modification, also termed deimination, is catalyzed by a group of enzymes called the protein arginine deiminases (PADs). In mammals, there are five PAD family members (i.e., PADs 1, 2, 3, 4, and 6) that exhibit tissue-specific expression patterns and vary in their subcellular localization. The kinetic characterization of PAD4 was recently reported, and these efforts guided the development of the two most potent PAD4 inhibitors (i.e., F- and Cl-amidine) known to date. In addition to being potent PAD4 inhibitors, we show here that Cl-amidine also exhibits a strong inhibitory effect against PADs 1 and 3, thus indicating its utility as a pan PAD inhibitor. Given the increasing number of diseases in which dysregulated PAD activity has been implicated, the development of PAD-selective inhibitors is of paramount importance. To aid that goal, we characterized the catalytic mechanism and substrate specificity of PADs 1 and 3. Herein, we report the results of these studies, which suggest that, like PAD4, PADs 1 and 3 employ a reverse protonation mechanism. Additionally, the substrate specificity studies provided critical information that aided the identification of PAD3-selective inhibitors. These compounds, denoted F4- and Cl4-amidine, are the most potent PAD3 inhibitors ever described.
Biochemistry 2006 oct
Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization.
Abstract
Abstract
Protein arginine deiminase 4 (PAD4) is a transcriptional coregulator that catalyzes the calcium-dependent conversion of specific arginine residues in proteins to citrulline. Recently, we reported the synthesis and characterization of F-amidine, a potent and bioavailable irreversible inactivator of PAD4. Herein, we report our efforts to identify the steric and leaving group requirements for F-amidine-induced PAD4 inactivation, the structure of the PAD4-F-amidine x calcium complex, and in vivo studies with N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide (Cl-amidine), a PAD4 inactivator with enhanced potency. The PAD4 inactivators described herein will be useful pharmacological probes in characterizing the incompletely defined physiological role(s) of this enzyme. In addition, they represent potential lead compounds for the treatment of rheumatoid arthritis because a growing body of evidence supports a role for PAD4 in the onset and progression of this chronic autoimmune disorder.