PLX4032
MEK/ERK pathway inhibitor; Inhibits B-RAF
概要
PLX4032 is an ATP-competitive inhibitor of the serine/threonine kinase B-RAF proto-oncogene, with IC₅₀ values of 31 and 100 nM for the wild-type and V600E mutant forms respectively (Khazak et al.; Sala et al.).
CANCER RESEARCH
· Inhibits proliferation in colon, melanoma, and thyroid carcinoma cancer cell lines expressing B-RAF V600E, alone or in synergy with taxol, vinblastine, and oxaliplatin compounds (Khazak et al.).
· Suppresses MEK and ERK phosphorylation downstream of B-RAF in melanoma cells with mutations at the V600 position, correlated with antiproliferative effects (Joseph et al.; Yang et al.).
· Inhibits tumor growth in B-RAF V600E melanoma tumor xenograft models (Yang et al.).
CANCER RESEARCH
· Inhibits proliferation in colon, melanoma, and thyroid carcinoma cancer cell lines expressing B-RAF V600E, alone or in synergy with taxol, vinblastine, and oxaliplatin compounds (Khazak et al.).
· Suppresses MEK and ERK phosphorylation downstream of B-RAF in melanoma cells with mutations at the V600 position, correlated with antiproliferative effects (Joseph et al.; Yang et al.).
· Inhibits tumor growth in B-RAF V600E melanoma tumor xenograft models (Yang et al.).
Alternative Names
RG-7204; Ro 51-85426; Vemurafenib
Cell Type
Cancer Cells and Cell Lines
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research
CAS Number
918504-65-1
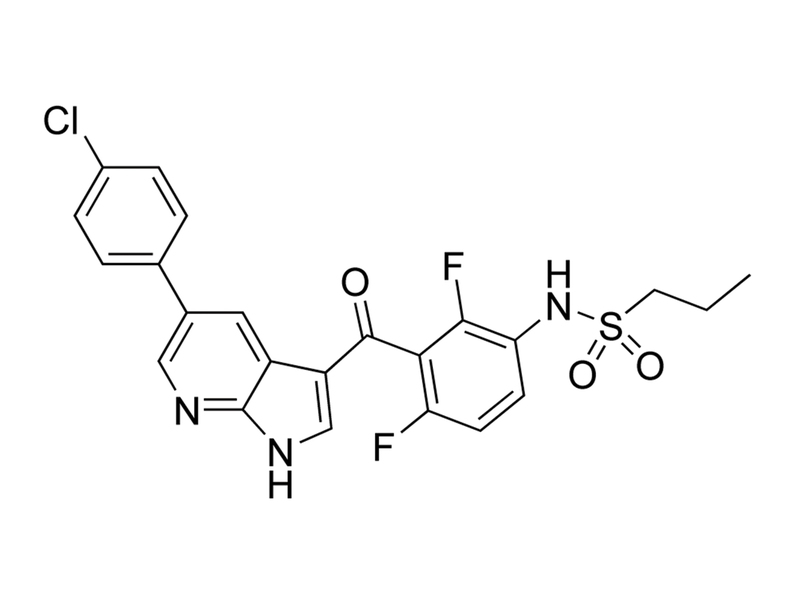
Chemical Formula
C₂₃H₁₈ClF₂N₃O₃S
Molecular Weight
489.9 g/mol
Purity
≥ 98%
Pathway
MEK/ERK
Target
B-RAF
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | PLX4032 | 73332, 73334 | All | English |
| Safety Data Sheet | PLX4032 | 73332, 73334 | All | English |
数据及文献
Publications (4)
Cancer research 2010 JUL
RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models.
Abstract
Abstract
The BRAF(V600E) mutation is common in several human cancers, especially melanoma. RG7204 (PLX4032) is a small-molecule inhibitor of BRAF(V600E) kinase activity that is in phase II and phase III clinical testing. Here, we report a preclinical characterization of the antitumor activity of RG7204 using established in vitro and in vivo models of malignant melanoma. RG7204 potently inhibited proliferation and mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase and ERK phosphorylation in a panel of tumor cell lines, including melanoma cell lines expressing BRAF(V600E) or other mutant BRAF proteins altered at codon 600. In contrast, RG7204 lacked activity in cell lines that express wild-type BRAF or non-V600 mutations. In several tumor xenograft models of BRAF(V600E)-expressing melanoma, we found that RG7204 treatment caused partial or complete tumor regressions and improved animal survival, in a dose-dependent manner. There was no toxicity observed in any dose group in any of the in vivo models tested. Our findings offer evidence of the potent antitumor activity of RG7204 against melanomas harboring the mutant BRAF(V600E) gene.
Proceedings of the National Academy of Sciences of the United States of America 2010 AUG
The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner.
Abstract
Abstract
Tumors with mutant BRAF and some with mutant RAS are dependent upon ERK signaling for proliferation, and their growth is suppressed by MAPK/ERK kinase (MEK) inhibitors. In contrast, tumor cells with human EGF receptor (HER) kinase activation proliferate in a MEK-independent manner. These findings have led to the development of RAF and MEK inhibitors as anticancer agents. Like MEK inhibitors, the RAF inhibitor PLX4032 inhibits the proliferation of BRAF(V600E) tumor cells but not that of HER kinase-dependent tumors. However, tumors with RAS mutation that are sensitive to MEK inhibition are insensitive to PLX4032. MEK inhibitors inhibit ERK phosphorylation in all normal and tumor cells, whereas PLX4032 inhibits ERK signaling only in tumor cells expressing BRAF(V600E). In contrast, the drug activates MEK and ERK phosphorylation in cells with wild-type BRAF. In BRAF(V600E) tumor cells, MEK and RAF inhibitors affect the expression of a common set of genes. PLX4032 inhibits ERK signaling output in mutant BRAF cells, whereas it transiently activates the expression of these genes in tumor cells with wild-type RAF. Thus, PLX4032 inhibits ERK signaling output in a mutant BRAF-selective manner. These data explain why the drug selectively inhibits the growth of mutant BRAF tumors and suggest that it will not cause toxicity resulting from the inhibition of ERK signaling in normal cells. This selectivity may lead to a broader therapeutic index and help explain the greater antitumor activity observed with this drug than with MEK inhibitors.
Molecular cancer research : MCR 2008 MAY
BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells.
Abstract
Abstract
BRAF-activating mutations have been reported in several types of cancer, including melanoma ( approximately 70% of cases), thyroid (30-70%), ovarian (15-30%), and colorectal cancer (5-20%). Mutant BRAF has constitutive kinase activity and causes hyperactivation of the mitogen-activated protein kinase pathway. BRAF silencing induces regression of melanoma xenografts, indicating the essential role of BRAF for cell survival. We set up an inducible short hairpin RNA system to compare the role of oncogenic BRAF in thyroid carcinoma versus melanoma cells. Although BRAF knockdown led to apoptosis in the melanoma cell line A375, the anaplastic thyroid carcinoma cell ARO underwent growth arrest upon silencing, with little or no cell death. Reexpression of the thyroid differentiation marker, sodium iodide symporter, was induced after long-term silencing. The different outcome of BRAF down-regulation in the two cell lines was associated with an opposite regulation of p21(CIP1/WAF1) expression levels in response to the block of the BRAF mitogenic signal. These results were confirmed using a specific BRAF small-molecule inhibitor, PLX4032. Restoration of p21(CIP1/WAF1) expression rescued melanoma cells from death. Altogether, our data indicate that oncogenic BRAF inhibition can have a different effect on cell fate depending on the cellular type. Furthermore, we suggest that a BRAF-independent mechanism of cell survival exists in anaplastic thyroid cancer cells.
Expert opinion on therapeutic targets 2007
Selective Raf inhibition in cancer therapy.
Abstract
Abstract
Over the past 5 years, the Raf kinase family has emerged as a promising target for protein-directed cancer therapy development. The goal of this review is to first provide a concise summary of the data validating Raf proteins as high-interest therapeutic targets. The authors then outline the mode of action of Raf kinases, emphasizing how Raf activities and protein interactions suggest specific approaches to inhibiting Raf. The authors then summarize the set of drugs, antisense reagents and antibodies available or in development for therapeutically targeting Raf or Raf-related proteins, as well as existing strategies combining these and other therapeutic agents. Finally, the authors discuss recent results from systems biology analyses that have the potential to increasingly guide the intelligent selection of combination therapies involving Raf-targeting agents and other therapeutics.