RosetteSep™ Human B Cell Enrichment Cocktail
Immunodensity negative selection cocktail
概要
The RosetteSep™ Human B Cell Enrichment Cocktail is designed to isolate B cells from whole blood by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-B cells and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as RosetteSep™ DM-L (Catalog #15705) or Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The purified B cells are present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Isolated cells are untouched
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Isolated cells are untouched
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human B Cell Enrichment Cocktail (Catalog #15024)
- RosetteSep™ Human B Cell Enrichment Cocktail, 2 mL
- RosetteSep™ Human B Cell Enrichment Cocktail (Catalog #15064)
- RosetteSep™ Human B Cell Enrichment Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
B Cells
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human B Cell Enrichment Cocktail | 15024, 15064 | All | English |
| Safety Data Sheet | RosetteSep™ Human B Cell Enrichment Cocktail | 15024 | All | English |
| Safety Data Sheet | RosetteSep™ Human B Cell Enrichment Cocktail | 15064 | All | English |
数据及文献
Data
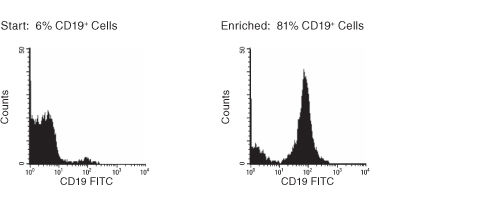
Figure 1. FACS Histogram Results With RosetteSep™ Human B Cell Enrichment Cocktail
Starting with fresh whole blood, the CD19+ cell content of the enriched fraction typically ranges from 81% - 83%.
Publications (39)
Frontiers in immunology 2020
Differential Expression of IgM and IgD Discriminates Two Subpopulations of Human Circulating IgM+IgD+CD27+ B Cells That Differ Phenotypically, Functionally, and Genetically.
Abstract
Abstract
The origin and function of blood IgM+IgD+CD27+ B cells is controversial, and they are considered a heterogeneous population. Previous staining of circulating B cells of healthy donors with rotavirus fluorescent virus-like particles allowed us to differentiate two subsets of IgM+IgD+CD27+: IgMhi and IgMlo B cells. Here, we confirmed this finding and compared the phenotype, transcriptome, in vitro function, and Ig gene repertoire of these two subsets. Eleven markers phenotypically discriminated both subsets (CD1c, CD69, IL21R, CD27, MTG, CD45RB, CD5, CD184, CD23, BAFFR, and CD38) with the IgMhi phenotypically resembling previously reported marginal zone B cells and the IgMlo resembling both na{\{i}}ve and memory B cells. Transcriptomic analysis showed that both subpopulations clustered close to germinal center-experienced IgM only B cells with a Principal Component Analysis but differed in expression of 78 genes. Moreover IgMhi B cells expressed genes characteristic of previously reported marginal zone B cells. After stimulation with CpG and cytokines significantly (p {\textless} 0.05) higher frequencies (62.5{\%}) of IgMhi B cells proliferated compared with IgMlo B cells (35.37{\%}) and differentiated to antibody secreting cells (14.22{\%} for IgMhi and 7.19{\%} for IgMlo). IgMhi B cells had significantly (p {\textless} 0.0007) higher frequencies of mutations in IGHV and IGKV regions IgMlo B cells had higher usage of IGHJ6 genes (p {\textless} 0.0001) and both subsets differed in their HCDR3 properties. IgMhi B cells shared most of their shared IGH clonotypes with IgM only memory B cells and IgMlo B cells with IgMhi B cells. These results support the notion that differential expression of IgM and IgD discriminates two subpopulations of human circulating IgM+IgD+CD27+ B cells with the IgMhi B cells having similarities with previously described marginal zone B cells that passed through germinal centers and the IgMlo B cells being the least differentiated amongst the IgM+CD27+ subsets."
Journal of immunology (Baltimore, Md. : 1950) 2019 may
Mechanism for IL-15-Driven B Cell Chronic Lymphocytic Leukemia Cycling: Roles for AKT and STAT5 in Modulating Cyclin D2 and DNA Damage Response Proteins.
Abstract
Abstract
Clonal expansion of B cell chronic lymphocytic leukemia (B-CLL) occurs within lymphoid tissue pseudofollicles. IL-15, a stromal cell-associated cytokine found within spleens and lymph nodes of B-CLL patients, significantly boosts in vitro cycling of blood-derived B-CLL cells following CpG DNA priming. Both IL-15 and CpG DNA are elevated in microbe-draining lymphatic tissues, and unraveling the basis for IL-15-driven B-CLL growth could illuminate new therapeutic targets. Using CpG DNA-primed human B-CLL clones and approaches involving both immunofluorescent staining and pharmacologic inhibitors, we show that both PI3K/AKT and JAK/STAT5 pathways are activated and functionally important for IL-15→CD122/ɣc signaling in ODN-primed cells expressing activated pSTAT3. Furthermore, STAT5 activity must be sustained for continued cycling of CFSE-labeled B-CLL cells. Quantitative RT-PCR experiments with inhibitors of PI3K and STAT5 show that both contribute to IL-15-driven upregulation of mRNA for cyclin D2 and suppression of mRNA for DNA damage response mediators ATM, 53BP1, and MDC1. Furthermore, protein levels of these DNA damage response molecules are reduced by IL-15, as indicated by Western blotting and immunofluorescent staining. Bioinformatics analysis of ENCODE chromatin immunoprecipitation sequencing data from cell lines provides insight into possible mechanisms for STAT5-mediated repression. Finally, pharmacologic inhibitors of JAKs and STAT5 significantly curtailed B-CLL cycling when added either early or late in a growth response. We discuss how the IL-15-induced changes in gene expression lead to rapid cycling and possibly enhanced mutagenesis. STAT5 inhibitors might be an effective modality for blocking B-CLL growth in patients.
Scientific reports 2019 mar
Computational analysis of the evolutionarily conserved Missing In Metastasis/Metastasis Suppressor 1 gene predicts novel interactions, regulatory regions and transcriptional control.
Abstract
Abstract
Missing in Metastasis (MIM), or Metastasis Suppressor 1 (MTSS1), is a highly conserved protein, which links the plasma membrane to the actin cytoskeleton. MIM has been implicated in various cancers, however, its modes of action remain largely enigmatic. Here, we performed an extensive in silico characterisation of MIM to gain better understanding of its function. We detected previously unappreciated functional motifs including adaptor protein (AP) complex interaction site and a C-helix, pointing to a role in endocytosis and regulation of actin dynamics, respectively. We also identified new functional regions, characterised with phosphorylation sites or distinct hydrophilic properties. Strong negative selection during evolution, yielding high conservation of MIM, has been combined with positive selection at key sites. Interestingly, our analysis of intra-molecular co-evolution revealed potential regulatory hotspots that coincided with reduced potentially pathogenic polymorphisms. We explored databases for the mutations and expression levels of MIM in cancer. Experimentally, we focused on chronic lymphocytic leukaemia (CLL), where MIM showed high overall expression, however, downregulation on poor prognosis samples. Finally, we propose strong conservation of MTSS1 also on the transcriptional level and predict novel transcriptional regulators. Our data highlight important targets for future studies on the role of MIM in different tissues and cancers.
Scientific reports 2019 jun
Elevated numbers of PD-L1 expressing B cells are associated with the development of AIDS-NHL.
Abstract
Abstract
The risk for non-Hodgkin lymphoma (NHL) is markedly increased in persons living with human immunodeficiency virus (HIV) infection, and remains elevated in those on anti-retroviral therapy (cART). Both the loss of immunoregulation of Epstein-Barr virus (EBV) infected cells, as well as chronic B-cell activation, are believed to contribute to the genesis of AIDS-related NHL (AIDS-NHL). However, the mechanisms that lead to AIDS-NHL have not been completely defined. A subset of B cells that is characterized by the secretion of IL10, as well as the expression of the programmed cell death ligand-1 (PD-L1/CD274), was recently described. These PD-L1+ B cells can exert regulatory function, including the dampening of T-cell activation, by interacting with the program cell death protein (PD1) on target cells. The role of PD-L1+ B cells in the development of AIDS-NHL has not been explored. We assessed B cell PD-L1 expression on B cells preceding AIDS-NHL diagnosis in a nested case-control study of HIV+ subjects who went on to develop AIDS-NHL, as well as HIV+ subjects who did not, using multi-color flow cytometry. Archival frozen viable PBMC were obtained from the UCLA Multicenter AIDS Cohort Study (MACS). It was seen that the number of CD19+CD24++CD38++and CD19+PD-L1+cells was significantly elevated in cases 1-4 years prior to AIDS-NHL diagnosis, compared to controls, raising the possibility that these cells may play a role in the etiology of AIDS-NHL. Interestingly, most PD-L1+ expression on CD19+ cells was seen on CD19+CD24++CD38++ cells. In addition, we showed that HIV can directly induce PD-L1 expression on B cells through interaction of virion-associated CD40L with CD40 on B cells.
Scientific reports 2019 jan
BCR-associated factors driving chronic lymphocytic leukemia cells proliferation ex vivo.
Abstract
Abstract
A chronic antigenic stimulation is believed to sustain the leukemogenic development of chronic lymphocytic leukemia (CLL) and most of lymphoproliferative malignancies developed from mature B cells. Reproducing a proliferative stimulation ex vivo is critical to decipher the mechanisms of leukemogenesis in these malignancies. However, functional studies of CLL cells remains limited since current ex vivo B cell receptor (BCR) stimulation protocols are not sufficient to induce the proliferation of these cells, pointing out the need of mandatory BCR co-factors in this process. Here, we investigated benefits of several BCR co-stimulatory molecules (IL-2, IL-4, IL-15, IL-21 and CD40 ligand) in multiple culture conditions. Our results demonstrated that BCR engagement (anti-IgM ligation) concomitant to CD40 ligand, IL-4 and IL-21 stimulation allowed CLL cells proliferation ex vivo. In addition, we established a proliferative advantage for ZAP70 positive CLL cells, associated to an increased phosphorylation of ZAP70/SYK and STAT6. Moreover, the use of a tri-dimensional matrix of methylcellulose and the addition of TLR9 agonists further increased this proliferative response. This ex vivo model of BCR stimulation with T-derived cytokines is a relevant and efficient model for functional studies of CLL as well as lymphoproliferative malignancies.
Scientific reports 2019 jan
Epstein-Barr Virus Latent Membrane Protein-1 Induces the Expression of SUMO-1 and SUMO-2/3 in LMP1-positive Lymphomas and Cells.
Abstract
Abstract
Epstein-Barr Virus latent membrane protein-1 (LMP1) interacts with the SUMO-conjugating enzyme Ubc9, which induces protein sumoylation and may contribute to LMP1-mediated oncogenesis. After analyzing human lymphoma tissues and EBV-positive cell lines, we now document a strong correlation between LMP1 and sumo-1/2/3 or SUMO-1/2/3 levels, and show that LMP1-induced sumo expression requires the activation of NF-kappaB signaling through CTAR1 and CTAR2. Together, these results point to a second mechanism by which LMP1 dysregulates sumoylation processes and adds EBV-associated lymphomas to the list of malignancies associated with increased SUMO expression.




