STEMdiff™ Cardiomyocyte Differentiation Kit
Media for differentiation of human PSCs to cardiomyocytes and long-term maintenance of human PSC-derived cardiomyocytes
概要
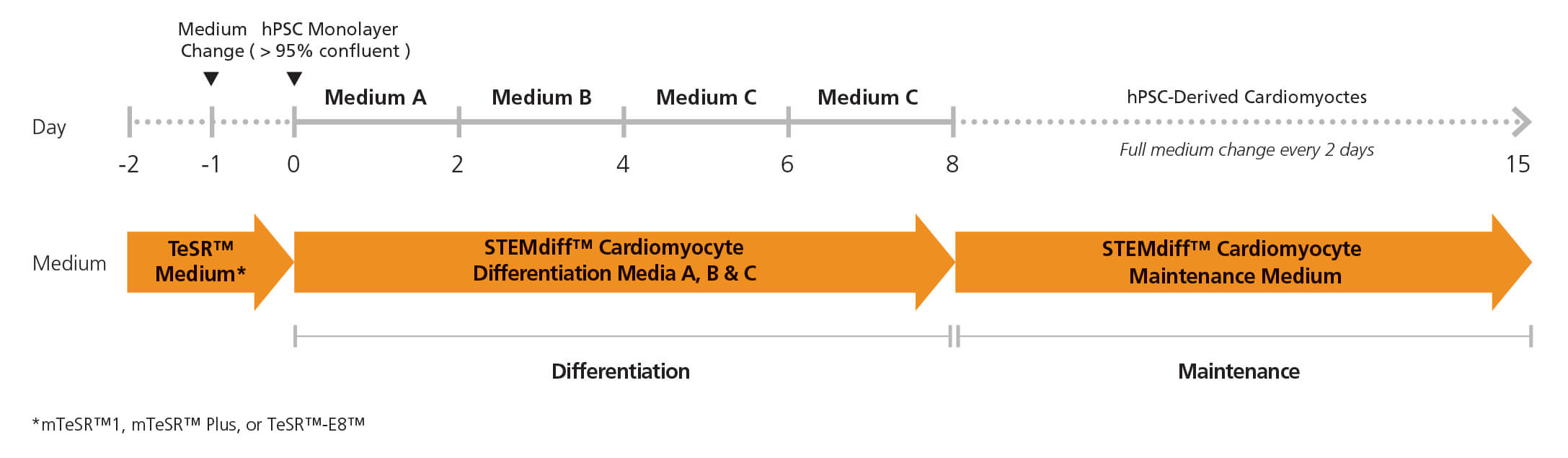
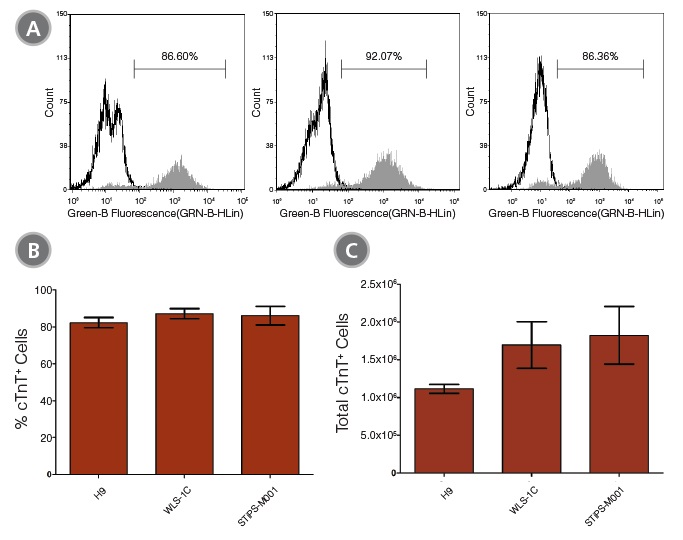
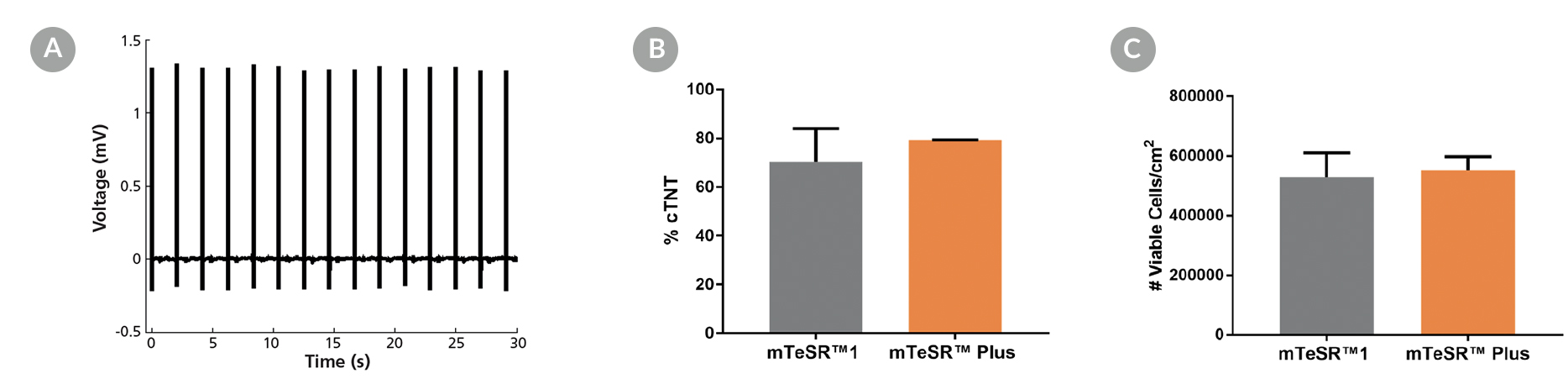
STEMdiff™ Cardiomyocyte Differentiation Kit (Catalog #05010) includes a medium for differentiation of human embryonic stem (ES) and induced pluripotent stem (iPS) cells (human pluripotent stem cells [hPSCs]) into cardiomyocytes (cardiac troponin T-positive [cTnT+]), as well as a medium for maintenance of hPSC-derived cardiomyocytes. This kit can be used to generate cardiomyocytes derived from a clump culture of hPSCs maintained in mTeSR™1 (Catalog #85850), mTeSR™ Plus (Catalog #100-0276), or TeSR™-E8™ (Catalog #05990). Greater than 80% of these cells will be cTnT+.
An average of 1 x 10^6 cells can be harvested from a single well of a 12-well plate.
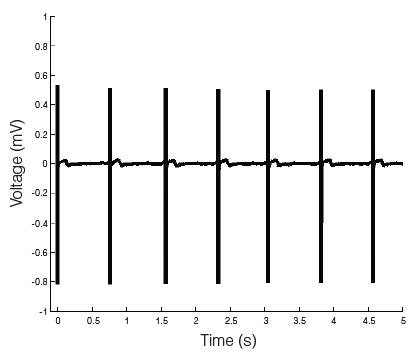
STEMdiff™ Cardiomyocyte Maintenance Kit (Catalog #05020) comprises the maintenance basal medium and supplement; it can be used for long-term maintenance of hPSC-derived cardiomyocytes for one month or longer. These cardiomyocytes can be used in various downstream applications and analyses.
• Supports the entire hPSC-derived cardiomyocyte workflow
• Simple monolayer protocol produces cardiomyocytes in 15 days
• One kit generates over 50 million cardiomyocytes (cTnT+)
• Robust performance with minimal variability across multiple hPSC lines
- STEMdiff™ Cardiomyocyte Differentiation Basal Medium, 380 mL
- STEMdiff™ Cardiomyocyte Differentiation Supplement A (10X), 10 mL
- STEMdiff™ Cardiomyocyte Differentiation Supplement B (10X), 10 mL
- STEMdiff™ Cardiomyocyte Differentiation Supplement C (10X), 20 mL
- STEMdiff™ Cardiomyocyte Maintenance Basal Medium, 490 mL
- STEMdiff™ Cardiomyocyte Maintenance Supplement (50X), 10 mL
Cardiomyocytes, PSC-Derived
Cell Culture, Differentiation, Maintenance
Disease Modeling, Drug Discovery and Toxicity Testing, Stem Cell Biology
数据及文献
Publications (12)
JACC. Basic to translational science 2020 may
Role of Blood Oxygen Saturation During Post-Natal Human Cardiomyocyte Cell Cycle Activities.
L. Ye et al.
Abstract
Blood oxygen saturation (SaO2) is one of the most important environmental factors in clinical heart protection. This study used human heart samples and human induced pluripotent stem cell-cardiomyocytes (iPSC-CMs) to assess how SaO2 affects human CM cell cycle activities. The results showed that there were significantly more cell cycle markers in the moderate hypoxia group (SaO2: 75{\%} to 85{\%}) than in the other 2 groups (SaO2 {\textless}75{\%} or {\textgreater}85{\%}). In iPSC-CMs 15{\%} and 10{\%} oxygen (O2) treatment increased cell cycle markers, whereas 5{\%} and rapid change of O2 decreased the markers. Moderate hypoxia is beneficial to the cell cycle activities of post-natal human CMs.
Cells 2020 jun
Extracellular Vesicles from Skeletal Muscle Cells Efficiently Promote Myogenesis in Induced Pluripotent Stem Cells.
D. Baci et al.
Abstract
The recent advances, offered by cell therapy in the regenerative medicine field, offer a revolutionary potential for the development of innovative cures to restore compromised physiological functions or organs. Adult myogenic precursors, such as myoblasts or satellite cells, possess a marked regenerative capacity, but the exploitation of this potential still encounters significant challenges in clinical application, due to low rate of proliferation in vitro, as well as a reduced self-renewal capacity. In this scenario, induced pluripotent stem cells (iPSCs) can offer not only an inexhaustible source of cells for regenerative therapeutic approaches, but also a valuable alternative for in vitro modeling of patient-specific diseases. In this study we established a reliable protocol to induce the myogenic differentiation of iPSCs, generated from pericytes and fibroblasts, exploiting skeletal muscle-derived extracellular vesicles (EVs), in combination with chemically defined factors. This genetic integration-free approach generates functional skeletal myotubes maintaining the engraftment ability in vivo. Our results demonstrate evidence that EVs can act as biological shuttles" to deliver specific bioactive molecules for a successful transgene-free differentiation offering new opportunities for disease modeling and regenerative approaches."
Cell reports 2020 jul
Modeling Type 1 Diabetes In Vitro Using Human Pluripotent Stem Cells.
N. C. Leite et al.
Abstract
Understanding the root causes of autoimmune diseases is hampered by the inability to access relevant human tissues and identify the time of disease onset. To examine the interaction of immune cells and their cellular targets in type 1 diabetes, we differentiated human induced pluripotent stem cells into pancreatic endocrine cells, including $\beta$ cells. Here, we describe an in vitro platform that models features of human type 1 diabetes using stress-induced patient-derived endocrine cells and autologous immune cells. We demonstrate a cell-type-specific response by autologous immune cells against induced pluripotent stem cell-derived $\beta$ cells, along with a reduced effect on $\alpha$ cells. This approach represents a path to developing disease models that use patient-derived cells to predict the outcome of an autoimmune response.
Leukemia 2020 jan
BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8+ cytotoxic T lymphocytes against multiple myeloma: clinical applications.
J. Bae et al.
Abstract
The purpose of these studies was to develop and characterize B-cell maturation antigen (BCMA)-specific peptide-encapsulated nanoparticle formulations to efficiently evoke BCMA-specific CD8+ cytotoxic T lymphocytes (CTL) with poly-functional immune activities against multiple myeloma (MM). Heteroclitic BCMA72-80 [YLMFLLRKI] peptide-encapsulated liposome or poly(lactic-co-glycolic acid) (PLGA) nanoparticles displayed uniform size distribution and increased peptide delivery to human dendritic cells, which enhanced induction of BCMA-specific CTL. Distinct from liposome-based nanoparticles, PLGA-based nanoparticles demonstrated a gradual increase in peptide uptake by antigen-presenting cells, and induced BCMA-specific CTL with higher anti-tumor activities (CD107a degranulation, CTL proliferation, and IFN-$\gamma$/IL-2/TNF-$\alpha$ production) against primary CD138+ tumor cells and MM cell lines. The improved functional activities were associated with increased Tetramer+/CD45RO+ memory CTL, CD28 upregulation on Tetramer+ CTL, and longer maintenance of central memory (CCR7+ CD45RO+) CTL, with the highest anti-MM activity and less differentiation into effector memory (CCR7- CD45RO+) CTL. These results provide the framework for therapeutic application of PLGA-based BCMA immunogenic peptide delivery system, rather than free peptide, to enhance the induction of BCMA-specific CTL with poly-functional Th1-specific anti-MM activities. These results demonstrate the potential clinical utility of PLGA nanotechnology-based cancer vaccine to enhance BCMA-targeted immunotherapy against myeloma.
Acta neuropathologica 2020
Loss of UGP2 in brain leads to a severe epileptic encephalopathy, emphasizing that bi-allelic isoform-specific start-loss mutations of essential genes can cause genetic diseases.
E. Perenthaler et al.
Abstract
Developmental and/or epileptic encephalopathies (DEEs) are a group of devastating genetic disorders, resulting in early-onset, therapy-resistant seizures and developmental delay. Here we report on 22 individuals from 15 families presenting with a severe form of intractable epilepsy, severe developmental delay, progressive microcephaly, visual disturbance and similar minor dysmorphisms. Whole exome sequencing identified a recurrent, homozygous variant (chr2:64083454A {\textgreater} G) in the essential UDP-glucose pyrophosphorylase (UGP2) gene in all probands. This rare variant results in a tolerable Met12Val missense change of the longer UGP2 protein isoform but causes a disruption of the start codon of the shorter isoform, which is predominant in brain. We show that the absence of the shorter isoform leads to a reduction of functional UGP2 enzyme in neural stem cells, leading to altered glycogen metabolism, upregulated unfolded protein response and premature neuronal differentiation, as modeled during pluripotent stem cell differentiation in vitro. In contrast, the complete lack of all UGP2 isoforms leads to differentiation defects in multiple lineages in human cells. Reduced expression of Ugp2a/Ugp2b in vivo in zebrafish mimics visual disturbance and mutant animals show a behavioral phenotype. Our study identifies a recurrent start codon mutation in UGP2 as a cause of a novel autosomal recessive DEE syndrome. Importantly, it also shows that isoform-specific start-loss mutations causing expression loss of a tissue-relevant isoform of an essential protein can cause a genetic disease, even when an organism-wide protein absence is incompatible with life. We provide additional examples where a similar disease mechanism applies.
Nature communications 2020
IGF1-mediated human embryonic stem cell self-renewal recapitulates the embryonic niche.
S. E. Wamaitha et al.
Abstract
Our understanding of the signalling pathways regulating early human development is limited, despite their fundamental biological importance. Here, we mine transcriptomics datasets to investigate signalling in the human embryo and identify expression for the insulin and insulin growth factor 1 (IGF1) receptors, along with IGF1 ligand. Consequently, we generate a minimal chemically-defined culture medium in which IGF1 together with Activin maintain self-renewal in the absence of fibroblast growth factor (FGF) signalling. Under these conditions, we derive several pluripotent stem cell lines that express pluripotency-associated genes, retain high viability and a normal karyotype, and can be genetically modified or differentiated into multiple cell lineages. We also identify active phosphoinositide 3-kinase (PI3K)/AKT/mTOR signalling in early human embryos, and in both primed and na{\{i}}ve pluripotent culture conditions. This demonstrates that signalling insights from human blastocysts can be used to define culture conditions that more closely recapitulate the embryonic niche."
View All Publications